| Issue |
BSGF - Earth Sci. Bull.
Volume 196, 2025
|
|
|---|---|---|
| Article Number | 6 | |
| Number of page(s) | 60 | |
| DOI | https://doi.org/10.1051/bsgf/2024027 | |
| Published online | 04 June 2025 | |
Coral biodiversity from Morocco after the End-Triassic mass extinction
Biodiversité corallienne du Maroc après l’extinction de masse du Trias-Jurassique
1
University of Geneva, Department of Earth Sciences, Rue des Maraîchers 13, 1205 Geneva, Switzerland
2
Université de Lorraine, CNRS, lab. GeoRessources, UMR 7359, BP 70239, 54506, Vandoeuvre-lès-Nancy Cedex, France
* Corresponding author: simon.boivin@unige.ch
Received:
20
January
2024
Accepted:
13
November
2024
Each new coral-bearing outcrop found in Lower Jurassic strata is useful to understand the evolution of corals between the end-Triassic mass extinction and the Toarcian anoxic event. Here we provide new taxonomic data on corals issued from fieldwork on four outcrops from the region of Amellagou, in the High Atlas Mountains, Morocco. A set of 157 coral specimens have been collected from a small biostrome, a giant reef and two olistholiths, spanning from Hettangian − Sinemurian time interval to early Pliensbachian. These corals are distributed in 14 families, 22 genera and 27 species. Among these species, two are new: Lepidophyllia (Heterastraea) microcalix sp. nov., represented enough to allow a population study and Paracuifia castellum sp. nov. The study extends the last appearance datum of several genera known only in the Triassic till now, namely: Parastraeomorpha, Araiophyllum, Paracuifia, Pinacophyllum and, possibly, Paravolzeia. For this reason, the severity of the end-Triassic mass extinction is questioned in comparison to the extinction events that happened around the Pliensbachian-Toarcian boundary. For this reason, moreover, the phyletic discontinuity between some Triassic and Jurassic taxa is also addressed. Some Lazarus taxa known from Triassic and Pliensbachian remain absent in Hettangian and now, at a lesser degree, in Sinemurian. That is why we assume that the absence of these taxa is only due to the poor preservation of coral environments during these times. This study also changes our view on the first appearance datum of several genera that were known in Jurassic strata, namely: Proleptophyllia, Vallimeandropsis and, possibly, Lochmaeosmilia. A special attention is given to the distribution of colonial arrangements and points to the important proportion of cerioid and solitary corals. Additionally, the study highlights the existence of significant proportions of thamnasterioid and meandroid forms. The presence of corals with such a level of integration, together with the occurrence of two species that show platy to ramose transition in their colony shape, namely Hispaniastraea murciana and Chrondrocoenia clavellata, stresses the effectiveness of a photosymbiosis in these Early Jurassic coral communities. Lastly, the proportion of solitary specimens increased over time, revealing the uniqueness of coral assemblages during the Pliensbachian.
Résumé
Chaque nouvel affleurement corallien découvert dans les strates du Jurassique inférieur est utile pour comprendre l’évolution des coraux entre l’extinction de la fin du Trias et l’événement anoxique du Toarcien. Nous fournissons ici de nouvelles données taxinomiques sur des récoltes de coraux issues de travaux de terrain sur quatre affleurements de la région d’Amellagou, dans les montagnes du Haut Atlas, au Maroc. Un ensemble de 157 spécimens de coraux a été collecté provenant d’un petit biostrome, d’un récif géant et de deux olistolithes s’étendant de l’intervalle de temps Hettangien −Sinémurien au Pliensbachien inférieur. Ces coraux sont répartis en 14 familles, 22 genres et 27 espèces. Parmi ces espèces, deux sont nouvelles, Lepidophyllia (Heterastraea) microcalix nov. sp., suffisamment représentée pour permettre une étude de population et Paracuifia castellum sp. nov. L’étude étend la dernière apparition de plusieurs genres connus jusqu’à présent seulement dans le Trias, à savoir : Parastraeomorpha, Araiophyllum, Paracuifia, Pinacophyllum, et peut-être Paravolzeia. Pour cette raison, la sévérité de l’extinction de la fin du Trias est remise en question par rapport aux événements d’extinction qui se sont produits autour de la limite Pliensbachien-Toarcien. Pour cette raison aussi, la discontinuité phylétique entre certains taxons du Trias et du Jurassique est également remise en question. Des taxons Lazare connus du Trias et du Pliensbachien restent absents dans l’Hettangien et maintenant à un moindre degré dans le Sinémurien. C’est pourquoi nous supposons que l’absence de ces taxons est uniquement due à la mauvaise préservation des milieux coralliens à ces époques. L’étude change notre point de vue sur la date de première apparition de plusieurs genres qui étaient connus dans les strates jurassiques, à savoir : Proleptophyllia, Vallimeandropsis, et peut-être Lochmaeosmilia. Une attention particulière est accordée à la distribution des arrangements coloniaux et souligne la proportion importante de coraux cérioïdes et solitaires. De plus, l’étude souligne l’occurrence de proportions non négligeables de formes thamnastérioïdes et méandroïdes. La présence de coraux avec un tel niveau d’intégration, ainsi que l’occurrence de deux espèces qui montrent une transition lamellaire à rameuse dans la forme de leur colonie (Hispaniastraea murciana et Chondrocoenia clavellata), indiquent l’efficacité d’une photosymbiose dans ces communautés coralliennes du Jurassique inférieur. La proportion de spécimens solitaires a augmenté avec le temps, ce qui indique la singularité des assemblages coralliens au cours du Pliensbachien.
Key words: Scleractinia / Morocco / Early Jurassic / extinction / biodiversity / recovery
Mots clés : Scleractinia / Maroc / Jurassique inférieur / extinction / biodiversité / récupération
© S. Boivin et al., Published by EDP Sciences 2025
 This is an Open Access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This is an Open Access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
1 Introduction
The beginning of the Early Jurassic is classically considered a difficult time for corals and therefore for reefal ecosystems in which corals have always played an important role throughout the Phanerozoic. This epoch was even referred to a “reef gap” (Flügel and Kiessling, 2002) or more softly to a “reef eclipse” (Stanley, 1992, 1997, 2001). The reality is that this period of reef disturbance occurs between two extinction events. Corals faced a first severe extinction at the end of the Triassic (Kiessling et al., 2007; Lathuilière and Marchal, 2009), then a second severe multiphased extinction at the end of the Pliensbachian (Vasseur et al., 2021). Consequently, the discovery of new coral records in the Hettangian, Sinemurian and Pliensbachian is still important for understanding corals evolution. The literature of course mentions corals in the Early Liassic for a long time in Western Europe (Orbigny, 1850; Martin, 1860; Dumortier, 1864; Fromentel and Ferry, 1865–1869; Duncan, 1865–1868; Terquem and Piette, 1868; Tomes, 1878, 1884a, 1888, 1893; Nicklès, 1902; Hahn, 1911; Joly, 1936). Part of these anciently known corals were revised by Alloiteau (1957) and Beauvais (1976) but new investigations have been undertaken more recently (Turnšek et al., 1975; Simms et al., 2002; Kiessling et al., 2009; Gretz et al., 2013, 2014; Gretz, 2014; Boivin et al., 2018). Early Jurassic corals have also been collected in Eastern Europe (Popa, 1981; Turnšek and Buser, 1999; Turnšek and Kosir, 2000; Turnšek et al., 2003), and a rather diverse fauna has been described from Central Asia (Melnikova, 1972, 1975a, 2006; Melnikova et al., 1993; Melnikova and Roniewicz, 2012, 2017) a part of which has been very recently reassigned to younger strata (Melnikova and Roniewicz, 2021).
Even if Lower Jurassic corals were also described from South America (Tilmann, 1917; Gerth, 1926, 1928; Wells, 1953; Prinz, 1991) and North America (Poulton, 1988; Stanley and McRoberts, 1993; Stanley and Beauvais, 1994; Hodges and Stanley, 2015), overall this is a difficult time for corals!
Africa has been poorly represented for a long and after some preliminary reports (Le Maître, 1935, 1937) the historical work of Beauvais (1986), devoted to the Liassic coral fauna of Morocco, appeared to be a keystone in our understanding of the group. However, the collection was mostly done in the Pliensbachian and Toarcian stages. Consequently, we undertook to revisit the field in Morocco taking advantage of the recent work of C. Durlet (unpublished), Sarih (2008) and Sarih et al. (2018) in the Amellagou region to investigate the Lower Jurassic successions.
Our recent fieldworks in Morocco, between 2014 and 2019, has already resulted in papers devoted to specific abundant taxa such as Hispaniastraea (Turnšek and Geyer, 1975; Boivin et al., 2019) and Neorylstonia Vasseur et al., 2019. This paper aims to establish a more complete record of early Liassic post-extinction corals. Following recent contributions involving European countries (Gretz et al., 2013, 2014; Boivin et al., 2018), the Moroccan outcrops in a favourable paleolatitudinal location offered a compelling opportunity to improve our knowledge of coral communities after the end-Triassic mass extinction.
2 Material and methods
A collection of coral specimens (157 samples) has been collected in the Amellagou region in the Moroccan High Atlas Mountains (Fig. 1). The material sampled is sourced from four Lower Jurassic outcrops presented in Table 1 and detailed below: the Dromedary biostrome (?Hettangian–Sinemurian), the Serdrar reef (upper Sinemurian), the Castle olistolith (upper Sinemurian or lowermost Pliensbachian) and the Owl olistolith (lower Pliensbachian).
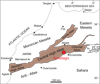 |
Fig. 1 Map of the main structural domains of Morocco with location of Amellagou Region in High Atlas Mountains. Modified after Krencker et al. (2014). |
In the field, benthic macrofossils were collected extensively, to obtain a complete survey of each reef outcrop. Samples were extracted with a hammer and chisel as well as with a battery-powered angle grinder, when necessary. The material was then photographed and studied under a reflected light stereomicroscope (Wild M5A). They were then cut and polished to obtain thin transverse and longitudinal sections of 70 µm thickness, and of 24 by 36 mm or of 40 by 50 mm as appropriate.
The samples were numbered according to the following code: the initials “SB” (for Simon Boivin) followed by a number given directly in the field (e.g., SB–133). Each piece collected sample is identified by a unique number (i.e., no batch including several samples). Thin sections made from the samples are identified by a letter next to the sample number (e.g., SB–133-a). In many cases, different coral species are included in the same sample. To distinguish them, a number preceded by a slash has been added (e.g., the specimens SB–133/1 and SB–133/2).
The silicified corals were extracted with hydrochloric acid. As the matrix is calcareous and the silica is not affected by HCl, the samples were immersed in acid (30%). The corals are thus isolated without their matrix.
The samples were studied mainly from thin sections due to their preservation, which is often strongly eroded over their entire natural surfaces. The thin sections are studied and imaged using a polarised microscope and a thin-section scanner. The description of the coral specimens was carried out following the exhaustive list of observable coral characters proposed by Beauvais et al. (1993); a re-structured version is available in Bertling (1995).
In the aim to study the morphometry of coral specimens, several measurements were taken thanks to a calliper or under a microscope with calibrated measurement software depending on the dimensions of specimens. Table 2 presents the different measurements made, also shown in a schematic representation in Figure 2. Depending on the morphology, the colonial structure and the preservation of the material, not all measurements could be taken.
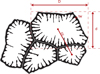 |
Fig. 2 Illustration of the main values measured for the morphometric study of corals. The black area corresponds to the skeleton. D calicular great diameter, d calicular small diameter, tw thickness of the wall, cc distance from corallite to corallite. |
The R software package was used to process the measurements and analyses (R Core Team, 2017) with the libraries ggplot2 (Wickham, 2009) for plots and iNext (Chao et al., 2014) for rarefaction curves. When sampling is large enough, the morphometric data were analysed with several Principal Component Analyses (PCA) with variance/covariance matrices. PAST software (Hammer et al., 2001) was also frequently used to visualize the data. All data are available in the appendices.
Presently, the samples are deposited at the Muséum d’Histoire Naturelle of Geneva, Switzerland. In the future, they should be redeposited at the Direction de la Géologie, Ministère de l’Énergie, des Mines et du Développement Durable − Département de l’Énergie et des Mines (here abbreviated to DGR), in Rabat, Morocco.
Summary table of outcrops sampled in the Amellagou Region, High Atlas Mountains, Morocco.
Main measurements made on coral specimens.
3 Systematics & results
Taxonomic names and synonymies are formulated according to the conventions proposed by Matthews (1973) and open nomenclature is used according to Bengtson (1988). Thus, synonymies are commented on using the following signs placed before the binoma:
* with the publication of this work, the species can be regarded as available under the terms of Article 11 of the International Code of Zoological Nomenclature (1999).
we accept the responsibility of linking the species to this reference.
(no sign) we have no reason to doubt the attachment of the species to this reference.
? we consider the allocation of this reference must be subject to some doubt.
non we do not attach the species to this reference.
v for "Vidimus", means that we have checked the deposited specimens that relate to the cited work.
p the reference is only partially applicable to the species under discussion.
Year in italics the species is mentioned without description or illustration.
All the species are described below and presented in systematic order. A summary is available in Table 3 with the number of specimens per location and the colonial arrangement of each genus. The specimens reported in synonymies concerning (Boivin, 2019) are the same specimens described and illustrated here.
List of genera and species with their distribution by localities and colonial structures. The numbers correspond to the numbers of specimens and brackets to doubtful assignations.
3.1 Abbreviations of institutions used
BRLSI Bath Royal Literary and Scientific Institution, United Kingdom.
BSPG Bayerische Staatssammlung für Paläontologie und historische Geologie, Munich, Germany.
DGR Direction de la Géologie, Ministère de l’Energie, des Mines et du Développement Durable − Département de l’Energie et des Mines, Rabbat, Morocco.
MHNG Muséum d’Histoire Naturelle of Geneva, Switzerland.
MNHN Muséum National d’Histoire Naturelle, Paris, France.
MSNP Museo di Storia Naturale dell’Università di Pisa, Calci, Italia.
Order Hexanthiniaria? Montanaro Gallitelli (1975)
Family Hispaniastraeidae (Boivin, Vasseur and Lathuilière (2019)
Genus Hispaniastraea Turnšek and Geyer (1975)
Type species. Hispaniastraea murciana Turnšek and Geyer (1975) by original designation.
Originally included species. Hispaniastraea murciana (Turnšek and Geyer, 1975) and Hispaniastraea ramosa (Turnšek and Geyer, 1975).
Etymology. Hispania from Spain in Latin.
Similarities and differences. The genus Hispaniastraea can be confused with the hypercalicified sponge genus Pseudoseptifer (Fischer, 1970) due to the similarities of their skeletons. A set of ten morphological features to distinguish these taxa is presented in Boivin et al. (2019).
Status. Available and valid
Hispaniastraea murciana Turnšek and Geyer (1975)
 |
Fig. 3 Hispaniastraea murciana Turnšek & Geyer, 1975. A Natural transverse section of the specimen SB–243. B Transverse thin section of a branch of the specimen SB–246. C–D Longitudinal thin sections of branches of the specimen SB–246. |
 |
Fig. 4 Hispaniastraea murciana Turnšek & Geyer, 1975. A Transverse thin section of a branch of the specimen SB–54. B Transverse thin section a branch of the specimen SB–85. C Longitudinal thin section of the specimen SB–169. D Longitudinal thin section of a branch of the specimen SB–53. |
* 1975 Hispaniastraea murciana sp. nov. — Turnšek & Geyer in Turnšek et al., p. 138, Pl. 20 figs. 1–2, Pl. 21 figs. 1–2.
1975 Hispaniastraea ramosa sp. nov. — Turnšek & Geyer in Turnšek et al., p. 139, Pl. 22 figs. 1–3.
? 1980 Chaetetes (Pseudoseptifer) murciana (Turnšek & Geyer) — Beauvais, p. 30, Pl. 3 fig 2.
v. 1980 Blastochaetetes dresnayi sp. nov. — Beauvais, p. 33–35, figs. 6–7, Pl. IV fig. 2.
? 1991 Hispaniastraea ramosa Turnšek & Geyer — Prinz, p. 167, Pl. 2 fig. 5.
? 1994 Hispaniastraea ramosa Turnšek & Geyer — Senowbari-Daryan & Stanley, p. 52–53, fig. 5, Pl. 1 figs. 4–8.
? 2003 Hispaniastraea ramosa Turnšek & Geyer — Turnšek et al., p. 292–293, Pl. 3 figs. 1–4.
v. 2018 Hispaniastraea murciana Turnšek & Geyer — Vasseur, p. 216–217, fig. 3 .46.
v. 2019 Hispaniastraea murciana Turnšek & Geyer — Boivin etal., p. 8–10, figs. 4–5.
v. 2019 Hispaniastraea murciana Turnšek & Geyer — Boivin, p. 212, figs. 10.14 and 10.15.
v. 2021 Hispaniastraea murciana — Vasseur and Lathuilière, p. 1231, fig. 29.
Type designation. Holotype by original designation, col. no. 33, Turnšek et al. (1975), p. 138.
Type locality and horizon. Upper Sinemurian − lower Pliensbachian from Zarcilla de Ramos, Province of Murcia, Spain.
Originally included material. Three colonies (33a, 33b and 34) and two fragments of colonies (35 and 36) from the same horizon as the holotype of the Seyfried collection, reposited at the Departamento de Paleontología of the University of Granada (UG), Spain.
Etymology. murciana from province of Murcia (Spain).
Material examined. 17 specimens: SB–53/1, SB–54, SB–55/4, SB–64/4, SB–85, SB–141, SB–145/2, SB–161/2, SB–163, SB–169/3, SB–233, SB–243, SB–246, SB–247, SB–315, SB–336 and SB–346/2.
Ages and localities of material. Upper Sinemurian from Serdrar reef, upper Sinemurian or lowermost Pliensbachian from Castle olistolith and lower Pliensbachian from Owl olistolith, Amellagou Region, High Atlas Mountains, Morocco.
Description. Colonies massive in shape or ramose, cerioid. Transverse section of corallites is sub-circular or polygonal. Septal apparatus characterized by one major septum highly dominant in length and thickness, and up to eleven minor septa. The major septum is straight or sometimes curved, compact, without ornamentation apart from the auriculae on the inner margin of the septum and reaches the centre of the lumen. Inner margin of the major septum is enlarged at the level of the auriculae and rounded otherwise, the lumen is typically horseshoe-shaped. Minor septa are very short, often not discernible on the surface of the inner wall, compact, and their outline in transverse section varies from triangular to semi-circular. Inner margins of the minor septa with sharp granules that alternate in height with the auriculae of the major septum. Corallites could be connected by canals. Endotheca made of thin tabulae rarely preserved.
Dimensions. Calicular great diameter 0.45 to 1.38 mm — Calicular small diameter 0.14 to 1 mm — Diameter of branches 10 to 15.78 mm — Distance from corallite to corallite 0.5 to 2.1 mm — Thickness of the wall 0.09 to 0.84 mm — Width of major septum (at its middle) 51 to 574 µm — Length of major septum 100 to 600 µm — Number of visible septa 1 to 12 septa.
Similarities and differences. Hispaniastraea murciana (Turnšek and Geyer, 1975) differs from Hispaniastraea ousriorum (Boivin, Vasseur and Lathuilière, 2019) that shows one to four well-developed S1 septa in addition to the major septum.
Stratigraphic and geographic distribution. Sinemurian − lower Toarcian from Murcia (Spain), Middle and High Moroccan Atlas Mountains (Morocco), Languedoc (France), Calabria (Italia), as well as possibly North Chile and Peru, if the identifications of Prinz (1991) and Senowbari-Daryan and Stanley (1994) are confirmed.
Order Scleractinia (Bourne, 1900)
Family Actinastreidae (Alloiteau, 1952)
Genus Chondrocoenia (Roniewicz, 1989)
Type species. Prionastraea schafhaeutli (Winkler, 1861) by original designation.
Originally included species. Chondrocoenia schafhaeutli (Winkler, 1861), Chondrocoenia waltheri (Frech, 1890), Chondrocoenia ohmanni (Frech, 1890), Chondrocoenia sp. A and Chondrocoenia sp. B.
Similarities and differences. The genus Chondrocoenia differs from other Actinastreidae genera by its peritheca.
Etymology. Chondros a grain shaped object and koinos common from the granulated appearance of the coenosteum surface, both in ancient Greek.
Status. Available and valid.
Chondrocoenia clavellata (Terquem and Piette, 1865)
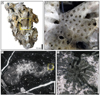 |
Fig. 5 Chondrocoenia clavellata (Terquem & Piette, 1865). A Specimen SB–194. B Enlargement of A. C Longitudinal thin section of branch of the specimen SB–49. D Enlargement of C. |
* 1865 Astrocoenia clavellata sp. nov. — Terquem & Piette, p. 130–131, Pl. 18 fig. 4–5.
1936 Astrocoenia clavellata Terquem & Piette — Joly, p. 172.
v. 2014 Chondrocoenia clavellata (Terquem & Piette) — Gretz, p. 99–102, Pl. 7–8.
v. 2018 Chondrocoenia clavellata (Terquem & Piette) — Boivin etal., fig. 3–c-d.
v. 2019 Chondrocoenia clavellata (Terquem & Piette) — Boivin, p. 188-189, fig. 10.2.
Type designation. Syntypes, no lectotype designated.
Type locality and horizon. Upper Hettangian − lower Sinemurian from Laval-Morency, Chilly, Maubert-Fontaine, Charleville and Saul, Northeastern France.
Originally included material. Unspecified. We did not localise the housing institution.
Etymology. From clava: club in Latin referring to the shape of branches.
Material examined. 3 specimens: SB–49, SB–194 and SB–318/2.
Ages and localities of material. Sinemurian from Dromedary biostrome and upper Sinemurian from Serdrar reef, Amellagou Region, High Atlas Mountains, Morocco.
Description. Ramose colonies, cerioid to plocoid. Transverse section of corallites sub-circular or polygonal. Radial elements are costosepta. Septa are thin, straight or curved, free or sometimes joined, confluent or sub-confluent, sub-compact. Septal apparatus ordered in two size orders. Lateral faces of septa are granulated. Inner margin of septa is rounded or slightly enlarged. Trabecular projections in the axial region. Columella styliform. Peritheca made of costae, dense, with granulated aspect in distal view of natural surface. Corallites inside branches are distributed in water-jet.
Dimensions. Calicular diameter 1 to 2 mm — Distance from corallite to corallite circa 0.9 to 1.7 mm — Septal density 6 to 8 septa per 1 mm.
Similarities and differences. See Chondrocenia martini below.
Stratigraphic and geographic distribution. Hettangian − Sinemurian from France, Belgium and Morocco (High Atlas Mountains).
Chondrocoenia martini (Fromentel, 1860)
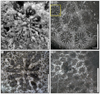 |
Fig. 6 Chondrocoenia martini (Fromentel, 1860). A Natural view of a calice of the specimen SB–343. B Transverse thin section of the specimen SB–343. C Enlargement of B. D Transverse thin section of the specimen SB–157. |
*v 1860 Stylastraea martini sp. nov. — Fromentel in Martin, p. 94, Pl. 7 fig. 18-19.
1861 Stylastraea martini Fromentel — Fromentel, p. 223.
1865 Stylastraea martini Fromentel — Terquem & Piette, p. 167.
1867 Astrocoenia plana sp. nov. — Duncan, p. 19, Pl. 5 fig. 1.
1867 Astrocoenia reptans sp. nov. — Duncan, p. 20, Pl. 4 figs. 4–5 and 15.
1867 Astrocoenia superba sp. nov. — Duncan, p. 21, Pl. 9 figs. 3–4.
1867 Cyathocoenia costata sp. nov. — Duncan, p. 29, Pl. 5 figs. 10–11.
1884b Stylastraea martini Fromentel — Tomes, p. 370.
1912 Stylastraea martini Fromentel — Lissajous, p. 179, pl. 18 fig. 54.
1917 Astrocoenia lissoni sp. nov. — Tilmann, p. 701, Pl. 26 fig. 4.
? 1953 Astrocoenia sp. cf. lissoni Tilmann —Wells, p. 3, fig. 1.
1976 Actinastraea plana (Duncan) — Beauvais, p. 44, Pl. 8 figs. 1–4, text-figs. 1–2.
1991 Actinastrea plana (Duncan) — Prinz, p. 161, Pl. 1 fig. 11.
1994 Actinastraea plana (Duncan) — Stanley & Beauvais, p. 43.
2013 Stylastraea martini Fromentel — Lathuilière, www.corallosphere.org with figures.
v. 2019 Chondrocoenia plana (Duncan) — Boivin, p. 190-191, fig. 10.3.
Type designation. Syntype. MHNG GEPI 036087.
Type locality and horizon. Hettangian (initially described in the Ammonites moreanus zone), from Vic de Chassenay, Côte d’Or, France.
Originally included material. Unspecified.
Etymology. Dedicated to Jules Martin.
Material examined. 2 specimens: SB–157 and SB–243.
Ages and localities of material. Upper Sinemurian or lowermost Pliensbachian from Castle olistolith and lower Pliensbachian from Owl olistolith, Amellagou Region, High Atlas Mountains, Morocco.
Description. Colonies cerioid to plocoid. Massive in shape. Transverse section of corallite circular to sub-circular. Septa are thick, straight, free or joined, compact, non-confluent. Septal apparatus organized in two size orders, typically eight to twelve S1 and a variable number of S2. S2 septa are thinner than S1 septa. Septal junctions are very regular: S2 septa are joined to neighbouring S1 septa. Lateral faces of S1 and S2 septa are ornamented with granules. Septa are enlarged at outer margin that give to corallite a multi-lobed aspect. Columella styliform. Extra-calicular budding. Radial symmetry very marked in smaller corallites. Endotheca made of dissepiments.
Dimensions. Calicular diameter 0.9 to 3.45 mm — Distance from corallite to corallite 2 to 3 mm — Septal density 3 to 4 septa per 1 mm — Number of septa circa 20 septa.
Similarities and differences. This species is close from Chondrocoenia clavellata (Terquem and Piette, 1865) but differs by its lower septal density and the colonial form.
Stratigraphic and geographic distribution. Hettangian from Chile and Great Britain, Sinemurian from Canada, Sinemurian − Pliensbachian from Morocco (High Atlas Mountains), Middle Liassic of Peru.
Genus Lochmaeosmilia (Wells, 1943)
Type species. Stylosmilia trapeziformis (Gregory, 1900) by original designation.
Originally included species. Lochmaeosmilia aethiopica (Wells, 1943), Lochmaeosmilia koniakensis (Ogilvie, 1897) and Lochmaeosmilia trapeziformis (Gregory, 1900).
Similarities and differences. The genus Lochmaeosmilia is characterised by its interconnecting apophyses, a character that is known in another genus i.e., Apocladophyllia (Morycowa and Roniewicz, 1990), a coral known in Toarcian (Vasseur et al., 2021) and in the interval Kimmeridgian-Berriasian. According to Morycowa and Roniewicz (1990), the septal apparatus is regular radial and bi-radial in Apocladophyllia, and particularly irregular and anastomosing in Lochmaeosmilia. The microstructure is said to be different due to minute and dense granules in Apocladophyllia that have not been observed in Lochmaeosmilia.
Etymology. From lochme: thicket, copse, especially as the lair of wild beast, in ancient Greek referring to the branching form of the colony, and smilia: scalpel biter also in ancient Greek.
Status. Available and valid.
Lochmaeosmilia? sp.
 |
Fig. 7 Lochmaeosmilia? sp. Transverse thin section of the specimen SB–155. |
Material examined. 1 colony: SB–155.
Ages and localities of material. Upper Sinemurian or lowermost Pliensbachian from Castle olistolith, Amellagou Region, High Atlas Mountains, Morocco.
Description. Branching colony with high density of calices, extensively recrystallised. Septal apparatus almost not visible (Fig. 7). Branches are regularly connected witch each other by apophyses.
Dimensions. Diameter of corallites 0.3 mm — 3 to 4 branches per mm2 — Number of septa: 12 (approximated from a quarter of calix) — Septal density: 2 septa per 100 µm.
Remarks. The preservation of our single specimen does not allow a formal identification, despite it is probably a new species. The diagnostic characters of the septa were not observed, that is why we put a question mark after the genus name.
Similarities and differences. Our specimen can be distinguished from other nominal species of Lochmaeosmilia by the low size of its corallites (Tab. 4). This is also valid for species of Apocladophyllia (Tab. 4).
Stratigraphic and geographic distribution. Sinemurian − lowermost Pliensbachian from Morocco (High Atlas Mountains). The extension of the genus which was considered Middle Jurassic in age (Beauvais, 1971), is probably much wider.
FamilyAstraeomorphidae (Frech, 1890)
Genus Parastraeomorpha (Roniewicz, 1989)
Type species. Parastraeomorpha minuscula (Roniewicz, 1989) by original designation.
Originally included species. P. minuscula (Roniewicz, 1989) and P. similis (Roniewicz, 1989).
Similarities and differences. This genus is close to Astraeomorpha (Reuss, 1854) but differs by the lack of menianae and the development of thick synapticulae with oblique axes whereas they are horizontal in Parastraeomorpha.
Etymology. From para: near in ancient Greek, referring to the morphological similarity to Astraeomorpha (Reuss, 1854).
Status. Available and valid.
Parastraeomorpha minuscula (Roniewicz, 1989)
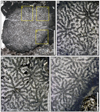 |
Fig. 8 Parastraeomorpha minuscula Roniewicz, 1989. A Transverse thin section of the specimen SB–344. B–C–D Enlargement of A. |
p 1890 Astraeomorpha confusa Winkler. var. nov. minor — Frech, p. 68, Pl. 19 figs. 4, 11 and 12 (non fig. 1, nec 7).
* 1989 Parastraeomorpha minuscula sp. nov. — Roniewicz, p. 98-99, Pl. 30 figs. 1–2.
2003 Parastraeomorpha minuscula Roniewicz — Stanley & Yarnell, p.114.
v. 2019 Parastraeomorpha minuscula Roniewicz — Boivin, p. 230-231, fig. 10.22.
Type designation. Holotype by original designation, NHMW 1982/57/17, (Roniewicz, 1989): p.98, Pl. 30 fig. 1.
Type locality and horizon. Rhaetian from Zlambach Beds of Fischerwiese, Northern Calcareous Alps, Austria.
Originally included material. Twenty-five fragments of colonies and four thin-sections.
Etymology. From minuscula: small in Latin referring to the small corallite dimensions.
Material examined. 1 colony: SB–344.
Ages and localities of material. Lower Pliensbachian from Owl olistolith, Amellagou Region, High Atlas Mountains, Morocco.
Description. Colony thamnasterioid with some aphroid tendencies, columnar. Corallites densely distributed without apparent organisation. Radial elements are bisepta or septa, confluent or not confluent, compact, often joined, flexuous. Septal apparatus hierarchized in two size orders. S1 septa are long and reach the columella, whereas S2 septa are joined to neighbouring S1 septa. Lateral faces of septa are ornamented with probable pennulae (the only available sections are not favourable for this observation). Symmetry hexameral well regular, indicating an intercalicular budding. Synapticula present. Axial area is occupied by a columella of unknown nature (perhaps parietal) and extremity of S1 septa. No wall.
Dimensions. Size of colony 9.4 × 11.3 mm — Calicular diameter circa 1 mm — Distance from corallite to corallite 0.9 to 1.2 mm — Number of septa circa 12–15 septa.
Similarities and differences. This species differs from Parastraeomorpha similis (Roniewicz, 1989) that has larger diameter of corallites, robust skeletal elements and a lamellar colony growth form.
Stratigraphic and geographic distribution. Rhaetian from Austria (Northern Calcareous Alps), Upper Triassic from Alaska, Pliensbachian from Morocco (High Atlas Mountains).
Family Comoseriidae (Fromentel, 1861) (= Microsolenidae Koby, 1889)
Genus Eocomoseris (Melnikova, Roniewicz and Löser, 1993)
Type species. Eocomoseris gurumdyensis nomen novum pro Eocomoseris ramosa Melnikova, 1993 non (Frech, 1890), p. 5, Pl. I fig. 1–3 by original designation. The type species is now considered to be junior synonym of Eocomoseris minima (Beauvais, 1986) by Vasseur and Lathuilière (2021, p. 1203).
Originally included species. Eocomoseris gurumdyensis Melnikova, 1993, Eocomoseris lamellata Melnikova, 1993, Eocomoseris raueni Loeser, 1993.
Similarities and differences. This genus seems very close to Spongiomorpha (Frech, 1890) that was placed among the Astraeomorphidae (Frech, 1890) (Roniewicz, 2010a). The connection between Astraeomorphidae and Comoseriidae remains a matter for future researches.
Etymology. Eos from dawn in ancient Greek and Comoseris referring to the comoseriid phylogeny.
Status. Available and valid.
Eocomoseris minima (Beauvais, 1986)
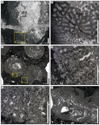 |
Fig. 9 Eocomoseris minima (Beauvais, 1986). A Longitudinal thin section of a branch of the specimen SB–222. B Enlargement of A. C Transverse thin section of branches of the specimen SB–245. D–E Enlargement of C. F Longitudinal thin sections of branches of the specimen SB–245. |
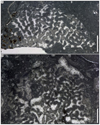 |
Fig. 10 Eocomoseris minima (Beauvais, 1986). A Longitudinal thin section of the specimen SB–167/2. B Longitudinal thin section of the specimen SB–316/2. |
P 1982 Actinastrea minima nomen nudum — Beauvais, p. 1964, pl. 1, fig. 3.
* 1986 Actinastrea minima sp. nov. — Beauvais, p. 31, Pl. 2 fig. 6, Pl. 6 fig. 6.
1993 Eocomoseris ramosa sp. nov. — Melnikova, p. 5, pl. I fig 1–3.
? 1994 Actinastraea minima Beauvais — Stanley & Beauvais, p. 42, fig. 6a–c (non fig. 6d).
2011 Eocomoseris gurumdyensis nomen novum pro Eocomoseris ramosa Melnikova — Roniewicz, p. 422.
2011 Eocomoseris ramosa (Frech, 1890) — Roniewicz, p. 424, fig. 7F.
v. 2019 Eocomoseris? cf. minima (Beauvais) — Boivin, p. 202-203, fig. 10.9–10.12.
2021 Eocomoseris gurumdyensis Melnikova in Roniewicz — Melnikova & Roniewicz, Table 1.
2021 Eocomoseris minima (Beauvais) — Vasseur & Lathuilière, p. 1203, fig. 9.
Remarks. Beauvais (1982) described under the name Actinastrea minima one specimen from Canada and designated another Moroccan specimen as a holotype, subsequently described in Beauvais (1986). The Canadian specimen is explicitly referred to a nomen nudum and the name has been available since 1986 (Articles 11 and 13 of the International Code of Zoological Nomenclature). We assign our material to the species defined by the Moroccan holotype (designated in (Beauvais, 1982) and described in (Beauvais, 1986) but we have reservation about the Canadian specimen that looks like a real actinastreid.
Type designation. Holotype MNHN.F.R11620, by original designation (Beauvais 1986, cf. remark above).
Type locality and horizon. Domerian from Jebel Bou Dahar, High Atlas Mountains, Morocco.
Originally included material. 9 specimens from the collection Menchikoff.
Etymology. minima from the small diameter of corallites.
Material examined. 16 specimens: SB–53/2, SB–64/1, SB–144/1, SB–146/2, SB–169/1, SB–198, SB–219/1, SB–222, SB–235, SB–245, SB–313, SB–317, SB–323 and SB–334.
Ages and localities of material. Upper Sinemurian from Serdrar reef, upper Sinemurian or lowermost Pliensbachian from Castle olistolith, and lower Pliensbachian from Owl olistolith, Amellagou Region, High Atlas Mountains, Morocco.
Description. Ramose colonies, branching or massive, thamnasterioid. Corallite small, vaguely delimited. Septa perforate, straight or curved, confluent. Septal apparatus organised in one size order. Septa are joined by two at their inner margin. Septa trabecular with pennulae, alternating with those of neighbouring septa. Columella styliform perhaps mono-trabecular (Fig. 9A). Synapticulae present.
Dimensions. Calicular diameter 1 to 2 mm — Diameter of branches 5 to 15 mm — Septal density 6 to 11 septa per 2 mm — Distance from corallite to corallite 1.2 to 1.8 mm — Width of pennulae circa 100 to 140 µm.
Similarities and differences. Eocomoseris minima differs from:
— Eocomoseris lamellata Melnikova, 1993 by its lamellar shape and well delimited corallites.
— Eocomoseris raueni Loeser, 1993 by its massive globular or flat shape and its larger corallites (Calicular diameter 2 to 3 mm and Distance from corallite to corallite 1.5 to 3.5 mm, according Melnikova et al., 1993).
Stratigraphic and geographic distribution. Norian from Austria, Sinemurian from Canada? Sinemurian − Pliensbachian from Morocco, Pliensbachian from Tajikistan.
Family Conophylliidae (Alloiteau, 1952)
Genus Araiophyllum (Cuif, 1975b)
Type species. Araiophyllum triasicum (Cuif, 1975b), p. 110–115, Pl. 16, figs. 17 and 18, by monotypy.
Originally included species. Only the type species.
Similarities and differences. This genus is close to genera Eocomoseris (Melnikova, Roniewicz and Löser, 1993) and Spongiomorpha (Frech, 1890) by its perforated nature, but it differs by its phaceloid nature.
Etymology. From araios: porous and phyllum: leaf, both in ancient Greek.
Status. Available and valid.
Araiophyllum triasicum (Cuif, 1975b)
 |
Fig. 11 Araiophyllum triasicum (Cuif, 1975b). Transverse thin section of the specimen SB–242/2. |
* 1975b Araiophyllum triasicum sp. nov. — Cuif, p. 110–115, p. 126, figs. 17–18, Pl. 16.
1989 Araiophyllum cf. triasicum Cuif — Turnšek & Buser, p. 86, Pl. 7 figs. 1–2.
1993 Araiophyllum triasicum Cuif — Cuif & Gautret, p. 407–408, Pl. 1 figs. 1–4.
1997 Araiophyllum triasicum Cuif — Turnšek, no 20.
v. 2019 Araiophyllum triasicum Cuif — Boivin, p. 186-187, fig. 10.1.
Type designation. Holotype by original designation (p. 115), MNHN.F.A79197.
Type locality and horizon. Carnian from Taurus Mountains, Lycia, Anatolia, Alakir Cay valley.
Originally included material. The text is ambiguous. Perhaps only one.
Etymology. From Triassic referring to the stratigraphic position of the material.
Material examined. 1 specimen: SB–242/2.
Ages and localities of material. Lower Pliensbachian from Owl olistolith, Amellagou Region, High Atlas Mountains, Morocco.
Description. Detached branch of a phaceloid coral. Radial elements are septa with pennulae without meniana, perforate, straight or slightly sinuous, joined. Septal apparatus organised in two size orders. S1 reach the papillose columella. S2 septa are short, sometimes contratingent on neighbouring S1 septa. Endotheca badly preserved, perhaps made of dissepiments. Perhaps pellicular epitheca.
Dimensions. Calicular great diameter 4.8 mm — Calicular small diameter 3.9 mm — Thickness of the wall circa 0.2 to 0.5 mm — Number of septa 25 or 26 septa — Septal density 3 to 4 septa per 1 mm.
Similarities and differences. Araiophyllum triasicum (Cuif, 1975b) differs from Araiophyllum liassicum (Beauvais, 1986) which has calices with a higher diameter (4 to 12 mm) and a greater septal density (12 septa per 2 mm).
Stratigraphic and geographic distribution. Carnian from Turkey (Taurus Mountains) and Slovenia (Pokljuka), Pliensbachian from Morocco (High Atlas Mountains).
Genus Thecactinastraea (Beauvais, 1986)
Type species. Thecactinastraea fasciculata (Beauvais, 1986) by original designation.
Originally included species. Only the type species.
Remarks. Thecactinastraea (Beauvais, 1986) and Phacelophyllia (Beauvais, 1986) are considered synonyms. In accordance with the principle of the first reviser (ICZN Art. 24.2) the priority is given to Thecactinastraea because the first synonymy was proposed in Melnikova and Roniewicz (2017), and not in Brame et al. (2019).
Similarities and differences. This genus differs from the phaceloid genera:
Paracuifia (Melnikova, 2001) that has poorly ornamented septa.
Paravolzeia (Roniewicz et al., 2005) that has poorly ornamented septa too.
Phacelostylophyllum (Melnikova, 1972) that is stylophyllid in septal structure.
Retiophyllia (Cuif, 1966) that is a distichophylliid in structure and shows a typical epitheca.
Etymology. From the genera Thecosmilia referring to the external morphology and Actinastraea referring to the structure of radial elements according to (Beauvais (1986).
Status. Available and valid.
Thecactinastraea fasciculata (Beauvais, 1986)
 |
Fig. 12 Thecactinastraea fasciculata Beauvais, 1986. A–C Transverse thin sections of the specimen SB–153. D Oblique thin section of the specimen SB–80. E Enlargement of D. F Oblique thin section of the specimen SB–80. |
* 1986 Thecactinastraea fasciculata sp. nov. —Beauvais, p.33, Pl. 5 fig. 1, text-fig. 21.
1986 Myriophyllum fasciatum sp. nov. — Beauvais, p.37, Pl. 9 fig. 1, text-fig. 25.
non 2003 Thecactinastraea fasciculata Beauvais — Turnšek et al., p.293, Pl.4 fig. 1-5.
2003 Phacelophyllia fasciata (Beauvais) — Turnšek et al., p.297, Pl.9 fig. 4.
2017 Thecactinastraea fasciculata Beauvais — Melnikova & Roniewicz, p. 133, fig. 3C, D.
v. 2018 Phacelophyllia fasciculata (Beauvais) — Vasseur, p. 279, fig. 3
v. 2019 Phacelophyllia fasciculata (Beauvais) — Boivin, p. 240, fig. 10
Remarks. Thecactinastraea fasciculata in Turnšek et al. (2003) is excluded of our synonymy because of the difference in the septal microstructure and microarchitecture similar to Cuifiidae (Melnikova, 1975b). Prinz (1991) considered Thecosmilia suttonensis (Duncan, 1867) to be a senior synonym of T. fasciculata Beauvais. We rather assign the Duncan’s species to the stylophyllid genus Phacelostylophyllum.
Type designation. Holotype by original designation, MNHN.F.R11611.
Type locality and horizon. Lower Jurassic from Tarhilest, near of Dayet Ifrah, Morocco.
Originally included material. 4 specimens from the collection du Dresnay, no. 4975 (1 specimen) and 4569 bis (3 specimens).
Etymology. From fasciculata: fasciculate in Latin.
Material examined. 2 colonies: SB–80 and SB–153.
Ages and localities of material. Upper Sinemurian or lowermost Pliensbachian from Castle olistolith, Amellagou Region, High Atlas Mountains, Morocco.
Description. Phaceloid colonies with a high density of branches. Transverse section of corallites sub-circular or deformed by the intracalicular budding. Radial elements are septa sub-compact, free, straight or slightly curved. Septal apparatus organised in three size orders hierarchized in length and thickness. S3 septa are very short and are not always visible (Fig. 12A). Septa pennular. Septa of all size orders show numerous trabecular detachments near the inner edge. Elongated fossa inducing a bilateral symmetry. Endotheca made of dissepiments. Peripheral wall.
Dimensions. Calicular great diameter 4.8 to 8.2 mm — Calicular small diameter 4.2 to 6.1 mm — Septal density 4 to 6 septa per 2 mm.
Similarities and differences. See T. termierorum (Beauvais, 1986) below.
Stratigraphic and geographic distribution. Upper Sinemurian − Toarcian from Morocco (Middle and High Atlas Mountains), Pliensbachian from Afghanistan, Domerian from Slovenia.
Thecactinastraea termierorum (Beauvais, 1986)
Figures 13 and 14
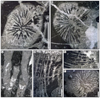 |
Fig. 13 Thecactinastraea termierorum (Beauvais, 1986). A–B Transverse thin section of the specimen SB–379. C Longitudinal thin section of the specimen SB–200. D–E Longitudinal thin section of the specimen SB–55/3. F Transverse thin section of the specimen SB–55/3. |
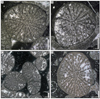 |
Fig. 14 Thecactinastraea termierorum (Beauvais, 1986). A–D Transverse thin sections of branches of the specimen SB–62. |
* 1986 Phacelophyllia termieri sp. nov. — Beauvais, p. 39, Pl. 7 fig. 2, text-fig. 27.
2003 Phacelophyllia termieri Beauvais — Turnšek et al., p. 297, Pl. 9 figs. 1–3.
2018 Phacelophyllia termieri Beauvais — Vasseur, p. 284-285, fig. 3.73.
v. 2019 Phacelophyllia termieri Beauvais — Boivin, p. 244, figs. 10.26–10.27.
Type designation. Holotype by original designation, MNHN.F.R11621.
Type locality and horizon. Lower Jurassic from Morocco (precise location unknown).
Originally included material. Only the holotype.
Etymology. Dedicated to Geneviève and Henri Termier. That is the reason why we emend the species name into a genitive plural (according to Articles 31.1.2 and 32.5 of the ICZN).
Material examined. 4 colonies: SB–55/3, SB–62, SB–200 and SB–379.
Ages and localities of material. Upper Sinemurian from Serdrar reef, Amellagou Region, High Atlas Mountains, Morocco.
Description. Colonies phaceloid with subparallel branches. Transverse section of corallites sub-circular or deformed by the intracalicular budding. Radial elements are septa sub-compact, joined, straight or curved. Septal apparatus organised in two or three size orders hierarchized in length but not in thickness. S1 septa are generally joined at their inner margins and reached the axial region. S2 septa are limited in length by the junction of the two neighbouring S1 septa. S3 septa are very short and are not always visible. Septa pennular. Few septa show trabecular detachments projecting into the axial region. Columella papillose. Epitheca made of dissepiments that can sometimes correspond on both side of a septum.
Dimensions. Calicular great diameter 5.9 to 7.7 mm — Calicular small diameter 4.5 to 7 mm — Septal density 5 to 8 septa per 2 mm. — Number of septa: 42 to 64 septa.
Similarities and differences. This species differs from Thecactinastraea fasciculata (Beauvais, 1986) that shows free septa and more trabecular detachments at inner margins of septa. P. termierorum differs from P. bacari (Turnšek, 2003) by the lower number of its septa and the higher size of its corallites.
Stratigraphic and geographic distribution. Lower Jurassic from Morocco, Upper Sinemurian from Morocco (High Atlas Mountains), Domerian from Slovenia, Toarcian from Morocco (High Atlas Mountains).
Family Cuifiidae (Melnikova, 1975b) (= Coryphyllidae Beauvais, 1981)
Remark on the family name. Melnikova (1975b) created the family Cuifiidae with Cuifia as a type genus and Cuifia gigantella as the type species of the type genus. Cuif (1975a) created the genus Coryphyllia, which he subsequently placed within his new family Distichophyllidae in Cuif (1977). Beauvais (1981) created a new family Coryphyllidae with Coryphyllia as a type genus. Roniewicz (1989) used the latter family erected by Beauvais but as a subfamily (Coryphyllinae) including it within Reimaniphyllidae (Melnikova 1975) (senior synonym of Distichophyllidae). However, Roniewicz and Stanley (2009) redrew the boundaries of the Coryphylliidae to include both Cuifia and Coryphyllia. This nomenclatural act that contradicts the principle of priority was subsequently followed by various authors (Roniewicz 2010c, Bo et al., 2017, Mannani 2020, Vasseur et al., 2021, Vasseur and Lathuilière 2021). Then we re-establish here the priority of Cuifiidae over Coryphylliidae.
Genus Coryphyllia (Cuif, 1975a)
Type species. Coryphyllia regularis (Cuif, 1975b) by original designation.
Originally included species. Only the type species.
Similarities and differences. Coryphyllia differs from:
- Cuifia (Melnikova, 1975b) by the structure of wall, epithecal in Coryphyllia whereas Cuifia shows a peculiar segmented structure of wall. In absence of microstructure preserved, these two genera are distinguished here by the differences of size orders (6 in Cuifia) and the number of septa (between 120–160 septa and septal density of 3–5 septa per 1 mm in Cuifia).
- Distichophyllia (Cuif, 1975c) that is characterised by a mid-septal line in zigzag, which is expressed in the morphology of thin septa. Moreover, Distichophyllia shows a septal apparatus hierarchized in thick S1 and S2 septa whereas following size orders septa are thin.
- Stylophyllopsis (Frech, 1890) that is characterised by septal spines joined by stereome, often producing detachments at the inner edge and a festooned transverse section in more outer position. The mid-septal line is very rare in Stylophyllopsis. The elongation of the fossa is also a good character for Coryphyllia.
Etymology. From coryphe: crest and phyllia: leaf both in ancient Greek.
Status. Available and valid.
Coryphyllia capillaria (Vasseur and Lathuilière, 2021)
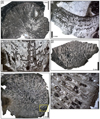 |
Fig. 15 Coryphyllia capillaria Vasseur & Lathuilière, 2021. A Transverse thin section of the specimen SB–244. B–C Longitudinal thin section of the specimen SB–230/1, section B distal and section C proximal. D Longitudinal thin section of the specimen SB–214. E Transverse thin section of the specimen SB–214. F Enlargement of E. Note the mid-septal line. |
v. 2018 Coryphyllia sp. 1 — Vasseur, p. 152-153, fig. 3.19.
v. 2019 Coryphyllia capillaria Vasseur & Lathuilière — Boivin, p. 192, fig. 10.4.
*v. 2021 Coryphyllia capillaria sp. nov. — Vasseur & Lathuilière, p. 1211, fig. 15.
Type designation. Holotype by original designation, CPUN PFPyr 7-2 in (Vasseur and Lathuilière 2021), the name was not available in Boivin (2019) ICZN art. 8.3.
Type locality and horizon. Pliensbachian from Estivère Pass, French Pyrenees.
Originally included material. Eight paratypes: CPUN 2303A1-2, CPUN 2303A8-1, CPUN AM16183-4, CPUN CDAm7, CPUN MA0504E7-13, CPUN MA0504E7-17, CPUN MA0704E3-10, CPUN MA0704E3-12.
Etymology. From capillus: hair in Latin referring to the very thin and long septa of high orders.
Material examined. 6 specimens: SB–230/1, SB–223, SB–244, SB–249, SB–341 and SB–346/1, and 1 uncertain silicified specimen: SB–68.
Ages and localities of material. Upper Sinemurian from Serdrar reef and lower Pliensbachian from Owl olistolith, Amellagou Region, High Atlas Mountains, Morocco.
Description. Solitary coral, conical, transverse section of corallite subcircular to slightly elliptical. Costosepta organised in a regular septal apparatus of three or four size orders difficult to distinguish. Septa are mostly straight or curved according to the bilateral symmetry, compact, free, slightly attenuated at inner margin. Lateral faces are weakly granulated. Endotheca made of abundant vesicular dissepiments regularly distributed in the interseptal space. Elongated fossa.
Dimensions. Calicular diameter 25 to 33 mm — Septal density 4 to 12 septa per 5 mm.
Similarities and differences. See C. regularis below.
Stratigraphic and geographic distribution. Sinemurian — Pliensbachian from Morocco (Middle and High Atlas Mountains), Pliensbachian from France (Pyrenees).
Coryphyllia regularis (Cuif, 1975a)
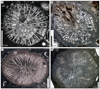 |
Fig. 16 Coryphyllia regularis Cuif, 1975a. A Transverse thin section of the specimen SB–69. B Transverse thin section of the specimen SB–146/1. C Transverse thin section of the specimen SB–316/1. D Transverse thin section of the specimen SB–242/1. |
* 1975a Coryphyllia regularis sp. nov. — Cuif, p. 380, fig 37 a–c, fig. 38.
1984 Coryphyllia regularis Cuif — Ramovš & Turnšek, p. 175, Pl. 4 fig. 1.
1989 Coryphyllia regularis Cuif — Turnšek & Buser, p. 84, Pl. 3.
1994 Coryphyllia regularis Cuif — Turnšek & Senowbari-Daryan, p. 481, Pl. 3 fig. 5.
2013 Coryphyllia regularis Cuif — Liao & Deng p. 54, Pl. 20 fig. 4.
v. 2018 Coryphyllia regularis Cuif — Vasseur, p. 194-195, fig. 10.5.
v. 2019 Coryphyllia regularis Cuif — Boivin, p. 194–195, figs. 10.5.
v. 2021 Coryphyllia regularis Cuif — Vasseur & Lathuilière, p. 1214, fig. 16.
Type designation. Holotype by original designation. MNHN.F.A31946; (Cuif, 1975a): p. 379, fig. 37 a, b? and c?
Type locality and horizon. Lower Norian from Valley Alakir Cay, Taurus Mountains, Turkey.
Originally included material. Cuif (1975a) specified that several specimens are included in the species but no further detail is given.
Etymology. From regularis in Latin referring to the regularity of the septal apparatus.
Material examined. 5 specimens: SB–69, SB–140, SB–146/1, SB–242/1 and SB–316/1 and 3 uncertain specimens: SB–145/1, SB–248 and SB–251.
Ages and localities of material. Upper Sinemurian from Serdrar reef, upper Sinemurian or lowermost Pliensbachian from Castle olistolith and lower Pliensbachian from Owl olistolith, Amellagou Region, High Atlas Mountains, Morocco.
Description. Solitary coral, transverse section of corallite circular to elliptical. Radial elements are septa (costae not visible) organised in a regular septal apparatus of three size orders. Septa are straight or curved, compact, free, sometimes contratingent, attenuated at inner margin, often rhopaloid. Lateral faces are ornamented with granules of low relief. Inner margin of septa is smooth. Vestiges of a wavy mid-septal line often visible. Columella absent, the fossa is tight and elongated (notably in elliptical corallite), defining the apparent bilateral symmetry of the corallite. Endotheca typical for this species, made of abundant vesicular dissepiments regularly distributed in the interseptal space.
Dimensions. Calicular great diameter 14.5 to 40 mm — Calicular small diameter 12.75 to 38 mm — Septal density 3 to 8 septa per 5 mm.
Similarities and differences. Coryphyllia regularis differs from:
- Coryphyllia capillaria (Vasseur and Lathuilière, 2021) by its lower number of septa, its lower septal density, and the size orders of its septal apparatus better hierarchized.
- Coryphyllia subregularis (Beauvais, 1986) that shows equivalent dimensions, differs by a less regular septal apparatus and larger dissepiments, and possibly a lacking epitheca.
- Coryphyllia bicuneiformis (Melnikova 1975 Vasseur and Lathuilière, 2021 Vasseur and Lathuilière, 2021) has a smaller diameter, more bicuneiform septa and a more elongated calice.
Stratigraphic and geographic distribution. Upper Triassic from China, Greece, Turkey and Slovenia, Sinemurian and Pliensbachian from Morocco (Middle and High Atlas Mountains).
Coryphyllia subregularis ? (Beauvais, 1986)
 |
Fig. 17 Coryphyllia subregularis ? Beauvais, 1986. Oblique thin section of the specimen SB–169/2. |
?p Stylophyllopsis mojsvari sp. nov. — (Frech, 1890), p. 52, Pl. 10 figs. 7—8, non figs. 9—14.
* 1986 Coryphyllia subregularis sp. nov. — Beauvais, p. 23, Pl. 4 fig. 3.
v. 2018 Coryphyllia subregularis Beauvais — Vasseur, p. 156–157, fig. 3.21.
v. 2019 Coryphyllia subregularis Beauvais — Boivin, p. 196, figs. 10.6.
2021 Coryphyllia subregularis Beauvais — Vasseur & Lathuilière, p. 1214, fig. 17.
Type designation. Holotype by original designation MNHN.F.R11609; Beauvais, p. 23, Pl. 4, fig. 3.
Type locality and horizon. Domerian, Fuciniceras cornacaldense horizon, from Jebel el Kounif (Bou-Arfa Range), Morocco.
Originally included material. No mentioned material other than the holotype.
Etymology. From sub: close to in Latin referring to the proximity with the species C. regularis.
Material examined. 1 uncertain specimen: SB–169/2.
Ages and localities of material. Upper Sinemurian or lowermost Pliensbachian from Castle olistolith, High Atlas Mountains, Morocco.
Description. Solitary coral. Septal apparatus regular. Septa are straight or curved, compact, sometimes contratingent, often rhopaloid. Endotheca made of large vesicular dissepiments regularly distributed in the interseptal space.
Dimensions. Septal density 4-5 septa per 5 mm.
Similarities and differences. This species is close to Coryphyllia regularis (Cuif, 1975a) and shows equivalent dimensions, but differs by a less regular septal apparatus and larger dissepiments. For comparison with other species, see C. regularis.
Stratigraphic and geographic distribution. Rhaetian from Austria? Upper Sinemurian − Pliensbachian from Morocco Middle and High Atlas Mountains.
Genus Paracuifia (Melnikova, 2001)
Type species. Protoheterastraea magnifica (Melnikova, 1984) by original designation, p. 44.
Originally included species. Paracuifia magnifica (Melnikova, 1984) and Paracuifia tortuosa (Melnikova, 2001).
Similarities and differences. Paracuifia is the single genus of Cuifiidae that shows phaceloid colonies. For comparisons with similar genera, see genus Thecactinastraea.
Etymology. From para: nearly in ancient Greek and cuifia referring to the genus Cuifia.
Status. Available and valid.
Paracuifia castellum sp. nov.
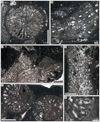 |
Fig. 18 Paracuifia castellum sp. nov. A & E transverse section of branches of the holotype SB–78/1. B–D longitudinal section of branches of the holotype SB–78/1. F Enlargement of E. |
Type designation. Holotype designated herein: SB–78/1 (20 thin sections: SB–78A to SB-78F, SB–78H to SB-78U).
Type locality and horizon. Upper Sinemurian or lowermost Pliensbachian from Castle olistolith, Amellagou Region, High Atlas Mountains, Morocco.
Originally included material. Only the holotype.
Etymology. From castellum: castle in Latin referring to the Castle olistolith named after the Berber ruins located just above the reef.
Diagnosis. Paracuifia with circular or slightly subcircular corallite about one centimetre in diameter, and around thirty septa.
Description. Colony phaceloid. Corallite circular or slightly subcircular. Septa thick, compact along the entire length, free or joined, straight or slightly undulated. Septal apparatus ordered in two size orders. Lateral margin of septa without ornamentation. Ghost of microstructure characterised by an aligned set of brown dots in the mid septal plan (Fig. 18F). Mid-septal line straight to slightly wavy (Fig. 18E). Endotheca made of large dissepiments regularly distributed in the interseptal space. These dissepiments show the same microstructure as septa (Fig. 18B), which indicated that the septa would be mostly made of thickening deposits. Columella absent. Costae absent. Probably trabeculothecal wall (Fig. 18E).
Dimensions. Calicular diameter 9.2 to 11.5 mm — Number of septa 30 septa — Septal density 2 to 3 septa per 3 mm.
Similarities and differences. Paracuifia castellum sp. nov. differs from:
— Paracuifia magnifica (Melnikova, 1984) that has an elliptic shape of corallites, a diameter from 6 mm in juvenile stage to 40 mm, and a number of septa around 200 in 5 size orders in adult stage.
— Paracuifia tortuosa (Melnikova, 2001) that has an elliptic shape of corallites, a diameter from 8 to 40 mm, and 130 to 150 septa in four size orders.
— Paracuifia smithi (Caruthers and Stanley, 2008) that has a very large and irregular corallite, a diameter from 10 to 23 mm, a tendency to be pseudo-meandroid, and poorly developed S2 septa.
— Paracuifia jennieae (Caruthers and Stanley, 2008) that has a pseudo-meandroid arrangement and a septal apparatus organised in four size orders.
— Paracuifia anomala (Caruthers and Stanley, 2008) that shows a cerioid arrangement of corallites.
Stratigraphic and geographic distribution. Upper Sinemurian or lowermost Pliensbachian from Morocco (High Atlas Mountains).
Family Dermosmiliidae (Koby, 1889)
Genus Proleptophyllia (Alloiteau, 1952)
Type species. Montlivaultia granulum (Fromentel and Ferry, 1866) by monotypy.
Similarities and differences. The genus Proleptophyllia differs from other Moroccan Lower Jurassic solitary genera by the moniliform character of the distal edge of septa.
Remarks. Following Vasseur and Lathuilière (2021), we have assumed the synonymy between Proleptophyllia and Epiphyllum (Alloiteau, 1957), and we have also included Cyclophyllopsis cornutiformis (Beauvais, 1986) in the synonymy of the species.
Etymology. From pro: before in ancient Greek and leptophyllia referring to the genus Leptophyllia (Reuss, 1854).
Status. Available and valid.
Proleptophyllia granulum (Fromentel and Ferry, 1866)
 |
Fig. 19 Proleptophyllia granulum (Fromentel & Ferry, 1866). A Transverse thin section of the specimen SB–342. B Enlargement of A showing the septal ornamentation. C Distal transverse section of the specimen SB–167/1. Proleptophyllia granulum? (Fromentel and Ferry, 1866). D Transverse section of the badly preserved specimen SB–326/1. |
*v. 1866 Montlivaultia granulum sp. nov. — Fromentel & Ferry, p. 136, pl. 24 fig. 2.
1866 Montlivaultia arenula sp. nov. — Fromentel & Ferry, p. 134-135, pl. 23 fig. 4.
1952 Proleptophyllia granulum (Fromentel & Ferry) — Alloiteau, p. 666.
1956 Proleptophyllia granulum (Fromentel & Ferry) — Alloiteau, Pal. univers. n∘ 121.
1957 Epiphyllum arenula (Fromentel & Ferry) — Alloiteau, p. 107, pl. 5 fig. 13.
1957 Proleptophyllia granulum (Fromentel & Ferry) — Alloiteau, p. 113, pl. 8 fig. 10, and fig. 73.
1986 Cyclophyllopsis cornutiformis nov. sp. — Beauvais, p. 34, pl. 7 fig. 1, pl. 8 fig. 1, text-fig. 23.
2011 Proleptophyllia cf. granulum (Fromentel & Ferry) — Lathuilière, p. 542, pl. 3 fig. 11–12.
.2018 Proleptophyllia granulum (Fromentel & Ferry) — Vasseur, p. 297–298, fig. 3.78.
v. 2019 Proleptophyllia granulum (Fromentel & Ferry) — Boivin, p. 254–255, figs. 10.31.
2021 Proleptophyllia granulum (Fromentel & Ferry) — Vasseur & Lathuilière, p. 1222, fig. 23.
Type designation. Lectotype MNHN.F.M03533, by inference from a holotype by Alloiteau (1956) (ICZN art 74.6).
Type locality and horizon. Pliensbachian from May-sur-Orne, Normandy, France.
Originally included material. Unspecified.
Etymology. From granulum: small grain in Latin referring to the shape of distal edge of septa.
Material examined. 2 specimens: SB–167/1 and SB–342 and 1 uncertain specimen: SB–326/1.
Ages and localities of material. Upper Sinemurian or lowermost Pliensbachian from Castle olistolith and lower Pliensbachian from Owl olistolith (and possibly upper Sinemurian from Serdrar reef), Amellagou Region, High Atlas Mountains, Morocco.
Description. Solitary coral. Transverse section of corallite sub-circular to slightly elliptical. Septa sub-compact with pennular structure, straight or slightly wavy, joined or contratingent. Hexameral symmetry. Septal apparatus organised in four size orders. Pennulae-like granules are not arranged in menianae and do not alternate between neighbouring septa. Trabecular axes are more and more detached toward the centre of corallite producing a parietal papillose columella.
Dimensions. Calicular diameter 18.7 to 19.7 mm — Number of septa 120 septa — Septal density 10 to 12 septa per 5 mm.
Stratigraphic and geographic distribution. Upper Sinemurian — lower Pliensbachian from Morocco, Pliensbachian from France, Domerian from Morocco, Toarcian from Morocco.
Family Latomeandridae (Alloiteau, 1952)
Genus Periseris (Ferry, 1870)
Type species. Agaricia elegantula (Orbigny, 1850) by monotypy.
Similarities and differences. This genus differs from Thamnasteria (Le Sauvage, 1823) by the septal structure, typically alternating pennular in Periseris with pennular rims turned upward. A complete comparison is available in Lathuilière (1990, p. 36). The differences with Thamnasteriamorpha (Melnikova, 1971), a Triassic genus, is still a matter of discussion (Lathuilière, 1990).
Etymology. From peri: around in ancient Greek and series: row in Latin probably referring to the meandroid tendency of the genus with concentric valleys.
Status. Available and valid.
Periseris elegantula (Orbigny, 1850)
 |
Fig. 20 Periseris elegantula (Orbigny, 1850). A Transverse thin section of the specimen SB–345. B Enlargement of A. C Longitudinal thin section of the specimen SB–345. D–E Enlargements of C. F Transverse thin section of the specimen SB–237. G Enlargement of F. |
* 1850 Agaricia elegantula sp. nov. — Orbigny, p. 293.
1990 Periseris elegantula (Orbigny) — Lathuilière, p. 38 Pl. 1–5, with synonymy (50 references).
1993 Periseris elegantula (Orbigny) — Pandey & Fürsich, p. 37, Pl. 11 fig. 2, text-fig 22.
2000 Periseris elegantula (Orbigny) — Lathuilière, p. 157–159, fig. 13.1–2.
2003 Periseris elegantula (Orbigny) — Pandey & Fürsich, p. 94, Pl. 26 fig. 1–6.
2005 Periseris elegantula (Orbigny) — Morycowa & Misik, p. 430, fig. 8.1–4.
? 2006 Periseris elegantula (Orbigny) — Pandey & Fürsich, p. 65, Pl. 5 fig. 4–7.
? 2011 Periseris sp. — Lathuilière, p. 540, Pl. II, figs. 5–9.
2018 Periseris elegantula — Vasseur, p. 277–278, fig. 3.70.
2019 Periseris sp. — Brame et al., fig. 7.F–G.
v. 2019 Periseris elegantula (Orbigny) — Boivin, p. 236-237, figs. 10.24.
v. 2023 Periseris elegantula (Orbigny) — Lathuilière et al., p. 30, fig. 9.C–D.
Type designation. Lectotype, by inference of a holotype by (Alloiteau, 1957) (ICZN art 74.6), MNHN.F.A26574.
Type locality and horizon. Bajocian from Langres, Haute-Marne, France.
Originally included material. Unspecified.
Etymology. From elegans: elegant and ula: a diminutive suffix in Latin.
Material examined. 2 colonies: SB–237 and SB–345.
Ages and localities of material. Lower Pliensbachian from Owl olistolith, Amellagou Region, High Atlas Mountains, Morocco.
Description. Flat or flattened hemispheric thamnasterioid colonies. Radial elements are biseptal sheets, thick, exactly confluent, subcompact, straight or sinuous, free or joined, sometimes contratingent. Pennular structure of radial elements, sub-continuous meniana with pennular rims turned upward (Fig. 20E,F). Pennulae of one septum alternate with those of the neighbouring septa. On both sides on a same septum, pennulae are not always aligned. Biseptal sheets show a sub-parallel preferential orientation between corallites (Fig. 20A). No hexameral symmetry visible. Endotheca badly preserved, perhaps made of vesicular dissepiments. Columella styliform. No wall. Remains of a holotheca are locally preserved (Fig. 20F).
Dimensions. Distance from corallite to corallite 3.6 to 7.8 mm — Septal density 4 to 6 septa per 2 mm.
Similarities and differences. Periseris elegantula is the only nominal species of the genus known for the Liassic. However, a Periseris sp. was described in Toarcian from Morocco (Lathuilière, 2011). It was kept in open nomenclature for its absence of holotheca.
Stratigraphic and geographic distribution. Pliensbachian and Toarcian from Morocco (High Atlas Mountains), Bajocian of France, Belgium, Luxembourg, Germany, Switzerland, Slovakia, Bathonian of India, Dogger from Iran.
The new discovery of such an ancient occurrence fills the gap between the genus Periseris and the closely related Triassic genus Thamnasteriamorpha (Melnikova, 1971) (=Thamnotropis Cuif, 1975b).
Family Protoheterastraeidae (Cuif, 1977)
Genus Paravolzeia (Roniewicz et al., 2005)
Type species. Paravolzeia alpina (Roniewicz et al., 2005) nomen novum pro Volzeia (Hexastraea) fritschi (Volz, 1896) in (Cuif, 1975a), non Hexastraea fritschi (Volz, 1896), by original designation.
Originally included species. Paravolzeia alpina (Roniewicz et al., 2005) and Paravolzeia timorica (Roniewicz et al., 2005).
Similarities and differences. This genus differs from the phaceloid genera:
- Paracuifia (Melnikova, 2001) that has a vesicular endotheca, and poorly hierarchised septa.
- Thecactinastraea (Beauvais, 1986) that has septa much more ornamented.
- Phacelostylophyllum (Melnikova, 1972) that is stylophyllid in septal structure.
- Retiophyllia (Cuif, 1966) that is a distichophylliid in structure and shows a typical epitheca.
Etymology. From para: near in ancient Greek, and volzeia referring to the similarity with the genus Volzeia (Cuif, 1966).
Status. Available and valid.
Paravolzeia? alpina? (Roniewicz et al., 2005)
 |
Fig. 21 Paravolzeia? alpina? Roniewicz et al., 2005. Transverse thin section of the specimen SB–219/2. |
1975a Volzeia (Hexastraea) fritschi sp. nov. — Cuif, p. 352, figs. 25-26 (non Hexastraea fritschi (Volz, 1896), p. 91, Pl. 11 figs. 14–20).
* 2005 Paravolzeia alpina sp. nov. — Roniewicz et al., p. 293. fig. 4–D, G.
2015 Paravolzeia alpina Roniewicz et al. — Stanley & Onoue, p. 21, fig. 15–a,b.
v. 2019 Paravolzeia? sp. — Boivin, p. 234, fig. 10.23.
Type designation. Roniewicz et al. (2005) strangely mentioned a lectotype for the creation of their own species. The "lectotype" would be housed in MNHN and implicitly referred to the material of Cuif (1975a). Unfortunately, in the Corallosphere website (Roniewicz, 2013), a holotype housed in Warsaw ZPAL HVIII/1 and figured in Roniewicz et al. (2005) fig. 4D, G is mentioned in contradiction with the previous designation.
Type locality and horizon. Carnian, Julian sub-stage, from Dolomites, northern Italy.
Originally included material. Only the type specimen?
Etymology. From alpina referring to the Alps.
Material examined. 1 sample: SB–219/2.
Ages and localities of material. Upper Sinemurian from Serdrar reef, Amellagou Region, High Atlas Mountains, Morocco.
Description. Fragment of colony, perhaps phaceloid. Septa thin, compact, free, sinuous. Septal apparatus organised in three size orders. S1 septa reach the centre of the corallite. Among them, some septa show a spoon-shaped inner margin, they could correspond to the six protosepta (?). S2 measure two thirds of the radius of the corallite in length. S3 are short and not always present. Lateral faces of septa lowly ornamented with possible granules. Fossa straight and narrow defining bilateral symmetry. Endotheca present made of few thin tabulae.
Dimensions. Calicular diameter circa 3 mm — Number of septa circa 30 septa extrapolated from the best-preserved sector — Septal density 3 septa per 1 mm.
Remarks. The only fragment found does not allow a firm identification.
Similarities and differences. Paravolzeia alpina (Roniewicz et al., 2005) differs from P. timorica (Roniewicz et al., 2005) that has a smaller calicular diameter (from 1.8 to 3 mm) and a poorly developped septal apparatus.
Stratigraphic and geographic distribution. Carnian from Dolomites (Italy), Norian from Japan, Sinemurian from Morocco?
Family Reimaniphylliidae (Melnikova, 1975b)
Genus Distichophyllia (Cuif, 1975a)
Type species. Montlivaltia norica (Frech, 1890): p. 39, pl. 3 fig. 8–9, pl. 10 fig. 1–5. pl. 18 fig. 7. by original designation.
Originally included species. Only the type species.
Similarities and differences. Differs from Coryphyllia by the microstructure of septa characterised by a mid-septal line in zigzag and fibrous, and laminar thickening deposits. In absence of preserved microstructure, the distinction remains difficult and we can suspect some inappropriate identifications (for instance in Deng and Zhang, 1984b). Moreover, Distichophyllia shows a septal apparatus hierarchized in thick S1 and S2 septa whereas following size orders septa are thin. Similar genera are compared in the paragraph similarities and differences of the genus Coryphyllia.
Etymology. From distichos: double row and phyllia: leaf both in ancient Greek, probably referring to usage in botany to designate a particular kind of phyllotaxy very similar to the structure of trabecular axes.
Status. Available and valid.
Distichophyllia norica (Frech, 1890)
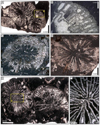 |
Fig. 22 Distichophyllia norica (Frech, 1890). A Transverse thin section of three contiguous corallites (specimens SB–202). B Enlargement of A. C Transverse thin section of the specimen SB–159. D Transverse thin section of the specimen SB–165. E Transverse thin section of the specimen SB–203. F Enlargement of E. |
1854 Montlivaltia cupuliformis n. sp. non Edwards & Haime — Reuss, p. 102, Pl. 6 figs. 16–17.
* 1890 Montlivaltia norica nomen novum — Frech, p. 39-40, Pl. 3 fig. 9, Pl. 10 figs. 1–5, Pl. 13 figs. 1–7, Pl. 18 fig. 17.
1890 Montlivaltia gosaviensis n. sp. — Frech, p. 41, Pl. 11 fig. 7.
1903 Montlivaltia aff. norica Frech — Kittl, p. 727 and 731.
? 1911 Montlivaltia norica Frech — Vinassa de Regny p. 99, Taf. 71, Fig. 15–17.
non 1911 Montlivaltia gigas sp. nov. (non Fromentel 1861) — Vinassa de Regny, p. 98, Pl. 70 figs. 12–13.
1927 Montlivaultia norica Frech — Smith, p. 126, Pl. 111 fig. 6.
1929 Montlivaltia norica Frech— Douglas, p. 645, PI. 46, figs. 1a, 1b, & 2.
1956 Montlivaltia norica Frech — Squires, p. 21, figs. 32–47.
? 1964 Montlivaltia sp. cf. M. norica Frech — Kanmera & Furukawa, p. 120, Pl. 12 figs. 6–10.
1966a Montlivaltia norica Frech — Kolosváry, p. 182.
1966b Monilivaltia norica Frech — Kolosváry, p. 127.
1975a Distichophyllia norica (Frech) — Cuif, p. 304-318, 398, figs. 2–6.
1975 Reimaniphyllia gosaviensis (Frech) — Melnikova, p. 87–89, Pl. 15 figs. 1 and ? 2.
? 1975 Montlivaltia norica Frech — Wu, p. 106, Pl. 4 figs. 6–7.
1977 Distichophyllia norica (Frech) — Cuif, p. 19, 39, fig. 4, Pl. 3 figs. 4–8, Pl. 4 figs. 5–7, Pl. 5 fig. 3.
p 1979 Montlivaltia norica Frech — Schäfer, p. 44, Pl. 10 fig. 1 ?, Pl. 11 fig. 2.
1979 "Montlivaltia" norica Frech — Stanley, p. 12, 24, 28, 32, 38.
? 1979 Disticophyllia cf. norica (Frech) — Montanaro Gallitelli, Russo, & Ferrari, p. 149, pl. 4 fig. 9 a–b.
1979 Montlivaltia norica Frech— Liao & Li, p. 53, Pl. 24 figs. 7–11.
? 1980 Montlivaltia norica Frech — Senowbari-Daryan, p. 39, Pl. 4 fig. 1.
? 1980 Montlivaltia norica Frech — Kristan-Tollmann et al., p. 173, Pl. 5 fig 6, Pl. 6 figs. 1, 3.
1980 Distichophyllia norica (Frech) — Cuif, p. 365, fig. 3.
1981 Montlivaltia norica Frech — Sadati, p. 199.
1982 Montlivaltia norica Frech — Berg & Cruz, p. 11.
? 1982 Montlivaltia norica Frech — Liao, p. 169, pl. 16 fig. 2a–c.
1982 Distichophyllia norica (Frech) — Buser et al., p. 21.
p 1984a Distichophyllia norica — Deng & Zhang, p. 261, Pl. 5 fig. 3? non fig. 2.
1984a Distichophyllia norica xizangensis (subsp. nov.) — Deng & Zhang, p. 261, Pl. 5 figs. 6–9.
1984a Montlivaltia tenuise (sp. nov.) — Deng & Zhang, p. 246, Pl. 2 figs. 3–6.
? 1984b Distichophyllia cf. norica — Deng & Zhang, p. 293, Pl. 4 fig. 3.
1985 Distichophyllia norica (Frech) — Bychkov & Melnikova, p. 161.
? 1986 Distichophyllia norica (Frech) — Stanley & Senowbari-Daryan, p. 173, fig. 3.
p 1986 Distichophyllia norica (Frech) — Stanley, p. 29, Pl. 3.1 figs. 4 and 6 non 5.
? 1986 Distichophyllia norica Frech — Xia & Liao, p. 44, Pl. 3 figs. 5–12.
1986 Distichophyllia cf. norica (Frech) —Melnikova & Bychkov, p. 65, Pl. 6 fig. 2,.
1986 Distichophyllia norica Frech — Iljina & Melnikova, p. 46, Pl. 12 fig. 1–2.
non 1986 Distichophyllia cf. norica Frech — Iljina & Melnikova, Pl. 18 fig. 1.
1987 Distichophyllia gosaviensis (Frech) — Turnšek & Ramovš, p. 35, Pl. 4 figs. 5–6.
1989 Distichophyllia norica (Frech) — Roniewicz, p. 39–41, Pl. 6 figs. 2–4.
? 1989 Distichophyllia norica (Frech) — Stanley & Whalen, p. 806–807, fig. 5.4 and 5.6.
1989 Distichophyllia norica (Frech) — Stanley, p. 770.
1990 Distichophyllia norica (Frech) — Riedel, p. 61.
? 1990 "Montlivaltia" verae (Volz) — Riedel, Pl. 11 fig. 4.
1991 Distichophyllia norica (Frech) — Turnšek & Buser, p. 227, Pl. 2 figs. 4–5.
1991 Distichophyllia norica (Frech) — Riedel, p. 114.
? 1993 Distichophyllum noricum (Frech) — Liao & Xia, p.207, fig. 2-3.
? 1994 Distichophyllia norica (Frech) — Stanley, p. 88, Pl. 4 figs. 3–4.
1994 Distichophyllia norica (Frech) — Liao & Xia, p. 54, Pl. 2 figs. 8–12, Pl. 3 fig. 8.
1994 Montlivaltia tenuise Deng and Zhang — Liao & Xia, p161, Pl. 67 figs. 2, 4–6.
? 1995 Distichophyllia norica (Frech) — Prinz-Grimm, p. 235, fig. 3c.
1996 Distichophyllia norica (Frech) — Bernecker, p. 54.
1996 Distichophyllia norica (Frech) — Senowbari-Daryan, p. 302.
1997 Distichophyllia norica (Frech) — Turnšek, p. 79, fig. 79.
1997 Distichophyllia gosaviensis (Frech) — Turnšek, p. 78, fig. 78
2000 Distichophyllia cf. norica — Blodgett et al., n° 9237
2001 Distichophyllia norica Frech — Melnikova, p. 46, Pl. 13 fig. 1.
2002 Distichophyllia cf. norica — McRoberts & Blodgett, p. 57.
2003 Distichophyllia norica (Frech) — Stanley & Yarnell, p 114.
2005 Distichophyllia norica (Frech) — Bernecker, p. 447 & 450.
2008 Distichophyllia norica (Frech) — Caruthers & Stanley, p.475, figs. 2.18–20, 2.25.
2009 Distichophyllia cf. norica (Frech) — Mannani & Yazdi, p. 368.
p 2010 Distichophyllia norica (Frech) — Rosenblatt, p. 99, Pl. 1 figs. 18–19, non Pl. 4 figs.15 & 19.
2012 Distichophyllia norica (Frech) — Shepherd et al., p. 807, fig. 6–1–6–3, 6–6, 6–7, 6–10 and 6–11.
non 2013 Distichophyllia norica (Frech) — Liao & Deng, p. 42, Pl. 10 figs. 7–10, Pl. 11 figs. 1–6.
2013 Distichophyllia norica xizangensis Deng & Zhang — Liao & Deng, p. 39, Pl. 6 figs. 11–14.
2013 Distichophyllia tenuise (Deng & Zhang) — Liao & Deng, p. 42, Pl. 11 figs. 7–11.
2017 Distichophyllia norica (Frech) — Bo et al., p. 272.
v. 2018 Distichophyllia sp. 2 — Vasseur, p.178-179, fig. 3.30.
v. 2019 Distichophyllia norica (Frech)— Boivin, p. 198, figs. 10.7 and 10.8.
? 2020 Distichophyllia cf. norica (Frech) — Mannani, p. 11, figs. 6-H and 6-I.
2020 Cuifia elliptica Melnikova — Mannani, p. 11, figs. 7-C —F.
v. 2021 Distichophyllia norica (Frech) — Vasseur & Lathuilière, p. 1244, fig. 40.
Type designation. Syntypes BSPG AS XII 46, 48, no lectotype designated.
Type locality and horizon. Norian — Rhaetian from Zlambach bei Aussee, Fischerwiese, Oedalm, Hammerkogel and Hallstätter Salzberg (Austria), and Scharitzkehl Alp (Germany).
Originally included material. The original type series included the specimen of Reuss (1854) and several hundred specimens collected by Frech (1890).
Etymology. From norica in Latin referring to the Noric Alps.
Material examined. 5 specimens: SB–55/5, SB–159, SB–165, SB–202 and SB–203 and 3 uncertain specimens: SB–138, SB–160/1 and SB–229.
Ages and localities of material examined. Upper Sinemurian from Serdrar reef and upper Sinemurian or lowermost Pliensbachian from Castle olistolith, Amellagou Region, High Atlas Mountains, Morocco.
Description. Solitary coral, corallum conical, transverse section of corallite circular to slightly elliptical. Radial elements are costosepta slightly bi-cuneiform or septa slightly attenuated, free, compact, wavy or zigzag. Septal apparatus is organised in 3 or 4 size orders. Septa of the first order are thick whereas septa of lower orders are thin. Lateral faces of septa are often ornamented by flattened, rounded or sharp granules. Inner margins of septa are rounded, slightly pointed or club-shaped, often reaching the centre of the corallite producing a parietal columella. The fossa is straight and elongated. General symmetry is hexameral and slightly bilateral due to the elongation of the fossa. Endotheca is made of large vesicular dissepiments regularly distributed in the interseptal space. Wall perhaps parathecal.
Dimensions. Calicular great diameter 10.5 to 30.5 mm — Calicular small diameter 14 to 24.5 mm — Number of septa 55 to 90 septa — Septal density 3 to 9 septa per 2 mm.
Similarities and differences. Distichophyllia norica differs from:
— Distichophyllia fritschi (Frech, 1890) that has strong protruding granulations of the lateral faces of its septa.
— Distichophyllia major (Roniewicz, 1996) that has a larger calice (between 35 to 50 mm), a denser septal apparatus (9 to 12 septa per 1 mm) and a higher length of S3 septa.
— Distichophyllia melnikovae (Montanaro Gallitelli, 1975) that has thicker septa. In the initial description, the distinction with D. norica is based on thickness of septa and number of septa (60 septa for D. melnikovae instead of 200 for D. norica according to Montanaro Gallitelli, Russo, and Ferrari, 1979). However, this low number appears included in the intra-specific variability of D. norica. It is possibly a junior synonym.
— Distichophyllia tabulata (Roniewicz, 1996) that has a larger calice (between 40 to 65 mm), tabuloid dissepiments and fusiform septa.
Stratigraphic and geographic distribution. Upper Triassic from China, Russia, Tibet, Carnian from Japan, Carnian − Norian from China and USA, Norian from Bosnia and Herzegovina, Canada, China, Iran, Oman, Peru, Romania, Russia and USA (including Alaska), Norian — Rhaetian from Austria, Chile, Iran, Tajikistan, Slovenia and Timor, Sinemurian − Pliensbachian from Morocco (High Atlas Mountains).
Genus Retiophyllia (Cuif, 1966)
Type species. Retiophyllia frechi (Roniewicz, 1989) nomen novum pro Thecosmilia fenestrata (Reuss, 1854) in (Frech, 1890) (p. 9, Pl. 1 figs. 25–27, Pl. 2 figs. 1–11 & 13–17 non 12) non Calamophyllia fenestrata (Reuss, 1854) (p. 105, Pl. 5 figs. 20–21). The type species was not properly designated in Cuif (1966: p. 130). The designation was corrected in Roniewicz (1989).
Originally included species. Only the type species.
Similarities and differences. The genus Retiophyllia (Cuif, 1966) differs from the phaceloid genera by its distichophylliid structure and its typical epitheca. This genus remains difficult to distinguish from Paracuifia (Melnikova, 2001) and Paravolzeia (Roniewicz et al., 2005). The shape of septa, the existence of costae, and remains of the mid septal zigzag line mostly guide the attribution. Thecactinastraea (Beauvais, 1986) has much more ornamented septa and Phacelostylophyllum (Melnikova, 1972) is stylophyllid in septal structure.
Etymology. Probably from the Rhaetian age and phyllia: leaf in ancient Greek.
Status. Available and valid.
Retiophyllia? cf. gracilis (Roniewicz, 1989)
 |
Fig. 23 Retiophyllia? cf. gracilis Roniewicz, 1989. A–B Transverse thin sections of two branches of the specimen SB–204/2. C. Enlargement of B. |
* 1989 Retiophyllia gracilis sp. nov. — Roniewicz, p. 58–59, Pl. 9 fig. 9, Pl. 10 fig. 2, Pl. 13 fig. 3.
1998 Retiophyllia gracilis Roniewicz — Roniewicz & Michalik, p. 396, fig. 1.1.
v 2009 Retiophyllia? cf. gracilis Roniewicz — Boivin, p. 256–257, fig. 10.32.
Type designation. Holotype by original designation, NHMW 1982/56/9.
Type locality and horizon. Norian — Rhaetian from Kesselwand-Rohrmoos, Zlambach Beds, Northern Calcareous Alps, Austria.
Originally included material. Several colonies and isolated corallites from Fischerwiese and Kesselwand-Rohrmoos, 8 thin sections.
Etymology. From gracilis: slim in Latin referring to the narrow corallite diameters.
Material examined. 1 specimen: SB–204/2.
Ages and localities of material. Upper Sinemurian from Serdrar reef, Amellagou Region, High Atlas Mountains, Morocco.
Description. Phaceloid colony. Radial elements are costosepta (Fig. 23C), compact, free, thin, straight, occasionally in zigzag. Septal apparatus organised in three size orders hierarchized in length but not in thickness. S1 septa reach the centre of calice. S2 septa measure circa three quarter of the radius of the calice. S3 are short. Lateral faces of septa are ornamented with sharp granules. The inner margin of S1 septa is slightly enlarged. Columella perhaps parietal. Endotheca made of thin dissepiments. The structure of the wall is not well preserved and remains difficult to decipher.
Dimensions. Diameter of branches circa 3.7 to 5.5 mm — Number of septa circa 40 septa — Septal density 6 septa per 2 mm — Distance between trabecular axes 120 to 200 µm.
Remarks. This specimen is referred to Retiophyllia gracilis (Roniewicz, 1989) based on its diameter, thickness and number of septa, and the organisation of size orders. However, distinctions between species of Retiophyllia are based mainly on the morphology of dissepiments, microstructure and dimensions (see (Roniewicz, 1989), Tab. 2 p. 44). In the lack of longitudinal section, it is impossible to confirm the identification.
Stratigraphic and geographic distribution. Rhaetian of Austria (Northern Calcareous Alps) and Slovakia (Western Carpathians) — Sinemurian from Morocco (High Atlas Mountains).
Family Stylophyllidae (Frech, 1890)
Genus Epismiliopsis (Alloiteau, 1952)
Type species. Epismilia liasica (Fromentel and Ferry, 1865b): p. 53, Pl. 15 fig. 3 by monotypy.
Originally included species. Only the type species.
Similarities and differences. The genus Epismiliopsis is very close to Stylophyllopsis Frech, 1890, even possibly congeneric, but differs by its elongated fossa and the torsion of its lateral septa. For other solitary genera, see Stylophyllopsis.
Etymology. From the genus Epismilia and opsis: appearance of in ancient Greek, referring to the similarity with the genus Epismilia.
Status. Available and valid.
Epismiliopsis sp.
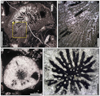 |
Fig. 24 Epismiliopsis sp. A Transverse thin section of the specimen SB–55/2. B Enlargement of A. C Transverse thin section of the specimen SB–331. D Enlargement of C. |
v p 2019 Stylophyllopsis? sp. — Boivin, p. 258, fig. 10.33.C–D, non fig. 10.33.A–B.
Material examined. 1 specimen: SB–331.
Ages and localities of material. Upper Sinemurian from Serdrar reef, Amellagou Region, High Atlas Mountains, Morocco.
Description. Solitary coral, conical corallum, transverse section of the corallite sub-circular. Septa with stylophyllid structure, free or sometimes joined. Septal apparatus organised in three or four size orders. Lateral septa of the first order are slightly bent. Inner margin of septa characterised by dissociated free septal spines, which are projected in the lumen. Septal spines are themselves ornamented according to the star-shape pattern described by Stolarski and Russo (2002, see Figs. 5 and 7). Fossa elongated. Endotheca made of dissepiments.
Dimensions. Calicular diameter 15 mm — Number of septa circa 60 septa (approximated).
Similarities and differences. We have compared our specimen to the nominal species of Epismiliopsis. Among them, E. conica (Beauvais, 1967) appears to be a Bajocian Montlivaltia. Four other nominal species are known: E. eudesi (Fromentel and Ferry, 1865b), E. liasicus (Fromentel and Ferry, 1865b), E. densserrae (Fromentel and Ferry, 1867), E. pareudesi (Vasseur and Lathuilière, 2021). The three latter species have too large dimensions in comparison to our specimen, which is close to E. eudesi but with a higher number of septa. However, E. eudesi is known by a single specimen, which could be a young corallum of E. pareudesi, as already noted by Vasseur and Lathuilière (2021). Accordingly, our specimen could also be a young corallum. Considering that it is also imperfectly preserved, we keep it in open nomenclature.
From the observation of the original figures (Terquem and Piette, 1865 Pl. 16 figs. 17–21), we cannot exclude an assignment to Epismiliopsis of Montlivaltia polymorpha (Terquem and Piette, 1865) because of the torsion of septa, and the elongation of the fossa.
Stratigraphic and geographic distribution. Sinemurian from Morocco (High Atlas Mountains).
Genus Lepidophyllia (Duncan, 1868)
Type species. Lepidophyllia hebridensis (Duncan, 1868) by subsequent designation (Wells, 1936): p. 114.
Originally included species. Lepidophyllia hebridensis (Duncan, 1868) and Lepidophyllia stricklandi (Duncan, 1868).
Etymology. From lepidos: blade and phyllia: leaf in ancient Greek.
Status. Available and valid.
Subgenus Heterastraea (Tomes, 1888
Type species. Isastræa tomesii (Duncan, 1868) by subsequent designation (Vaughan and Wells, 1943): p.156.
Originally included species. Isastræa murchisoni Wright in (Duncan, 1867), Septastræa eveshami (Duncan, 1868), Septastræa fromenteli (Terquem and Piette, 1865), Isastræa stricklandi (Duncan, 1868), Isastræa insignis (Duncan, 1868), Isastræa endothecata (Duncan, 1868), Septastræa haimei Wright in (Duncan, 1867), Isastræa tomesii Duncan, 1868), Isastræa latimæandroidea (Duncan, 1868), Septastræa excavata Fromentel in (Martin, 1860), Heterastræa etheridgei (Tomes, 1888), Heterastræa regularis (Tomes, 1888), Heterastræa bintonensis (Tomes, 1888) and Heterastræa sp. (Tomes, 1888). The species Isastræa sinemuriensis Fromentel in Martin, 1860) was included with doubt.
Similarities and differences. The subgenus Lepidophyllia (Heterastraea) differs from other cerioid taxa:
- Lepidophyllia (Lepidophyllia) Duncan, 1868) that is bifacial and has numerous rejuvenescences (Gretz et al., 2013).
- Rhaetiastraea (Roniewicz, 1974) by the non-trabecular nature of its septa (or at least the quasi exclusive proportion of thickening deposits in their microstructure), which implies a very limited ornamentation of lateral faces of septa. Moreover, no mid-wall line was described in Rhaetiastraea.
- Latiastrea (Beauvais, 1964), that has septa with pennular structure and endotheca made by vesicular dissepiments.
- Hispaniastraea (Turnšek and Geyer, 1975), that has a characteristic major septum.
- Chondrocoenia (Roniewicz, 1989), that is an actinastreid easily distinguished by its columella.
- Isastrea (Edwards and Haime, 1851b) that has a montlivaltid septal structure with carinae and a very different wall made of various components.
Etymology. From heteros: other in ancient Greek, probably referring to the other true Isastrea.
Status. Available and valid.
Lepidophyllia (Heterastraea) microcalix sp. nov.
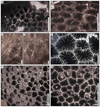 |
Fig. 25 Lepidophyllia (Heterastraea) microcalix sp. nov. A Transverse section of the colony SB–48. B Transverse section of the colony SB–204/1. C–D Transverse sections of the colony SB–210/1. G Transverse section of the colony SB–329. F Transverse section of the colony SB–211. G transverse section of the colony SB–318/1. |
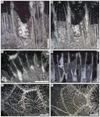 |
Fig. 26 Lepidophyllia (Heterastraea) microcalix sp. nov. A–B longitudinal sections of the colony SB–329. Note the two opposite septa cut longitudinally in the centre of the corallite and the ornamentation of their inner margins (A). C longitudinal section of the colony SB–228. D longitudinal section of the holotype SB–220. E–F transverse sections of the colony SB–216, note the septal intracalicular budding. |
 |
Fig. 27 Plots of morphometrical measurements of Lepidophyllia (Heterastraea) microcalix sp. nov. A Histograms of morphometrical measurements (calicular great diameter, calicular small diameter, thickness of the wall, distance from corallite to corallite, number of septa and septal density). B Morphospace plot of corallites from studied colonies computed with PCA (co-variance matrix) on the two calicular diameters (great and small), thickness of the wall and distance from corallite to corallite. Each dot color represents one colony. The two first principal components explain 92.9% of the total variance. |
v. 2019 Lepidophyllia (Heterastraea) microcalix (unavailable name, ICZN art. 8.3) — Boivin: p. 216, figs. 10.16, 10.17 and 10.18. 3.
Type designation. Holotype SB-220 (Fig. 26-D) and 30 paratypes: SB–48, SB–55/1, SB–57, SB–154, SB–193 (silicified), SB–196, SB–197, SB– 204/1, SB–210/1, SB–211, SB–212, SB–213, SB–216, SB–218/1, SB–224/1, SB–227, SB–228, SB–230/2, SB–250, SB–318/1, SB–319, SB–320, SB–321/1, SB–322, SB–325, SB–327, SB–329, SB–330, SB–333 and SB–340.
Type locality and horizon. Upper Sinemurian from Serdrar reef (sampling spot 2: N 32.00788° W 4.94478°), Amellagou Region, High Atlas Mountains, Morocco.
Ages and localities of paratypes. Dromedary biostrome (Hettangian or Sinemurian), Upper Sinemurian from Serdrar reef, Upper Sinemurian or lowermost Pliensbachian from Castle olistolith and Lower Pliensbachian from Owl olistolith — Amellagou Region, High Atlas Mountains, Morocco.
Etymology. From micro: small in ancient Greek and calix in Latin or kulix in ancient Greek: calyx, cup, referring to the small dimension of the calices.
Diagnosis. Lepidophyllia (Heterastraea) with small calices (see Tab. 5).
Description. Colonies cerioid. Corallites polygonal. Septal intracalicular budding (Figs 25–25, 26–E-F). Septa compact except the inner margin, free or sometimes joined (including contratingent junctions), confluent or not confluent, curved or wavy. Septal apparatus organised in three size orders, regularly hierarchized in length and thickness. Lateral faces of septa ornamented by fine granules. Spines are dissociated at inner margin of septa, orientation sub-horizontal of free spines (Fig. 26–A). The axial space is occupied by free spines. Depending on the length of septa and the depth of the transverse section, the axial region can be (1) a large fossa (Fig. 25–E), (2) occupied by a papillose columella made of free spines (Fig. 25–B) or (3) occupied by a parietal columella made of junction of septa (Fig. 26–E-F). No pali. Endotheca made of thin wide dissepiments occasionally steeply inclined (Fig. 26–A). Wall septothecal? Mid-wall line sometimes visible (Figs. 25–25–25, 26–E–F).
Dimensions. Dimensions are given in Table 5. The species present a moderate variability of the great and small diameters, the distance from corallite to corallite and the number of septa. The distribution of these dimensions is continuous (Fig. 27–A) and the morphospace is homogeneous (Fig. 27–B). The thickness of the wall and the septal density are still less variable. Consequently, we have no reason to split this species into several units.
Similarities and differences. Lepidophyllia (Heterastraea) microcalix has the smallest calices among nominal species of Lepidophyllia (Heterastraea) described in the literature (Tab. 6, Figs. 28 and 29): its diameter, distance from corallite to corallite and its number of septa are the smallest. Moreover, its septal density is higher than other species, except L. (H.) angelae. The PCA on the nominal species dimensions (Fig. 29) shows a clear separation between L. (H.) microcalix and other species on the second principal component (29.9% of total variance).
 |
Fig. 29 Morphospace plot of Lepidophyllia (Heterastraea) nominal species computed with PCA (co-variance matrix) on the dimensions from litterature presented in Table 6: calicular diameters (Dmin minimum value and Dmax maximum value), distance from corallite to corallite (CCmin minimum value and CCmax maximum value), number of septa (NSmin minimum value and NSmax maximum value) and septal density (SDmin minimum value and SDmax maximum value). The two first principal components explain 77.8% of total variance. |
Stratigraphic and geographic distribution. Hettangian? − Sinemurian − lower Pliensbachian from Morocco (High Atlas Mountains).
Genus Phacelostylophyllum (Melnikova, 1972)
Type species. Stylophyllopsis zitteli (Frech, 1890), p. 49, Pl. 13 figs. 9–15 & 17–24 by original designation.
Originally included species. Phacelostylophyllum? pontebbanae (Volz, 1896), Phacelostylophyllum romerloana (Volz, 1896), Phacelostylophyllum caespitosa (Frech, 1890), Phacelostylophyllum pygmaeum (Frech, 1890), Phacelostylophyllum zitteli (Frech, 1890), Phacelostylophyllum karauldyndalaensis (Melnikova, 1972).
Remarks. Here, Phacelostylophyllum has been described separately as a morphogenus defined by its phaceloid character but phyletically very close to Stylophyllopsis (Frech, 1890). That is why we choose to write Phacelostylophyllum mg. in conformity with the spectral nomenclature (Lathuilière, 1996).
Similarities and differences. The genus Phacelostylophyllum, stylophyllid in structure, differs from the phaceloid genera:
- Lochmaeosmilia (Wells, 1943) that shows typical apophyses.
- Paracuifia (Melnikova, 2001) and Paravolzeia (Roniewicz et al., 2005) which also have poorly ornamented septa, but do not show detachment of the septal spines at the distal inner edge.
- Thecactinastraea (Beauvais, 1986) that has much more ornamented septa.
- Pinacophyllum (Frech, 1890) that has complete tabulae.
- Retiophyllia (Cuif, 1966) that is a distichophyllid in structure and shows a typical epitheca.
Etymology. From phacelos: fascicle in ancient Greek and −stylophyllum referring to the genus Stylophyllum (Frech, 1890).
Status. Available and valid.
Phacelostylophyllum mg. martini (Fromentel, 1860)
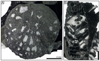 |
Fig. 30 Phacelostylophyllum mg. martini (Fromentel, 1860). A Transverse thin section of a branch of the specimen SB–205. B Longitudinal slightly oblique thin section of a branch of the specimen SB–231. |
v* 1860 Thecosmilia martini sp. nov. — Fromentel in Martin, p. 92, Pl. 8 fig. 8–9.
1861 Thecosmilia martini Fromentel — Fromentel, p. 142.
1864 Thecosmilia martini Fromentel — Dumortier, p. 95, Pl. 15 figs. 4, 6, 7.
1865 Thecosmilia martini Fromentel — Terquem & Piette, p. 127, Pl. 17 figs. 1–3.
1868 Thecosmilia martini Fromentel — Duncan, p. 45, Pl. 12 fig. 1–2.
1878 Thecosmilia martini Fromentel — Tomes, p. 186.
1884 Thecosmilia martini Fromentel — Koby, p. 164, Pl. 54 fig. 5.
1894 Thecosmilia martini Fromentel — Koby, p. 16, Pl. 4 fig. 3.
1936 Thecosmilia martini Fromentel — Joly, p. 170
? 1991 Phacelostylophyllum suttonensis (Duncan) — Prinz, p. 160–161, Pl. 1 fig. 10.
v. 2014 Phacelostylophyllum martini (Fromentel) — Gretz, p. 73, Pl. VI fig. e–f.
v. 2018 Phacelostylophyllum ?suttonensis (Duncan) — Boivin et al., fig. 3–e-g.
v. 2018 Phacelostylophyllum sp. 1 — Vasseur, p. 286, fig. 3.74.
v. 2019 Phacelostylophyllum martini — Boivin, p. 248, fig. 10.28.
v. 2021 Phacelostylophyllum mg. martini — Vasseur & Lathuilière, p. 1258, fig. 50.
Type designation. Holotype by monotypy. MHNG GEPI 36077.
Type locality and horizon. Sinemurian from Semur-en-Auxois, Burgundy, France.
Originally included material. Only the holotype.
Etymology. Dedicated to Jules Martin.
Material examined. 2 colonies: SB–205 and SB–231.
Ages and localities of material. Upper Sinemurian from Serdrar reef, Amellagou Region, High Atlas Mountains, Morocco.
Description. Colonies phaceloid. Transverse section of branches sub-circular. Radial elements are sub-compact, free, straight septa. Costae not observed. Septal apparatus organised in three size orders hierarchized in length. S1 septa are long and reach the axial region with perhaps projections. S2 septa measure circa a half of the radius. S3 are very short. Structure of septa perhaps stylophyllid, difficult to confirm due to the preservation. Lonsdaleoid septa. Endotheca made of large vesicular to tabular dissepiments (Fig. 30B). Wall nature unknown.
Dimensions. Diameter of branches 12 to 19 mm — Septal density 2 to 4 septa per 2 mm. — Endothecal density 2 to 3 dissepiments per 5 mm.
Similarities and differences. Phacelostylophyllum martini (Fromentel, 1860) differs from Phacelostylophyllum suttonensis (Duncan, 1867) that presents smaller calices (lower than 10 mm in diameter).
Stratigraphic and geographic distribution. Lower Jurassic from Western Europe, Hettangian from Scotland, Sinemurian from France (Languedoc) and Morocco (High Atlas Mountains), Pliensbachian from France (Pyrenees Mountains).
Phacelostylophyllum mg. suttonensis (Duncan, 1867)
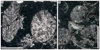 |
Fig. 31 Phacelostylophyllum mg. suttonensis (Duncan, 1867). A–B Transverse thin sections of branches of the specimen SB–170. |
* 1867 Thecosmilia suttonensis sp. nov. — Duncan, p. 11-12, Pl. IV figs. 7–9.
1884b Thecosmilia suttonensis Duncan — Tomes, p. 365.
non 1911 Calamophyllia suttonensis sp. nov. — Clapp & Shimer, p. 431, Pl. 40 figs. 5 and 7, Pl. 41 fig. 15.
1976 Phacelepismilia suttonensis (Duncan) — Beauvais, p. 68-69, Pl. XV fig. 1, text-fig. 34.
1983 Phacelepismilia suttonensis (Duncan) — Negus, p. 253.
non 1986 Thecactinastraea fasciculata sp. nov. — Beauvais, p. 33, Pl. 5 fig. 1, text-fig. 21.
non 1979 "Thecosmilia" cf. suttonensis (Clapp & Shimer) − Stanley, p. 34, Pl. 3 fig. 2.
non 1989 Retiophyllia suttonensis (Clapp & Shimer) − Stanley, p. 770, figs. 5–6.
1991 Phacelepismilia suttonensis (Duncan) — Negus, p. 255, Table 1
2002 Phacelepismilia suttonensis (Duncan) — Simms, Little, & Rosen, p. 34, figs. 6–7.
non 2009 Phacelophyllia suttonensis (Duncan) — Kiessling et al., p. 665, figs. 4D, 7F, 9A–C.
? 2009 Phacelostylophyllum cf. michelini (Terquem and Piette) — Kiessling et al., p. 666, fig. 9–f-g.
v. 2014 Phacelostylophyllum suttonensis (Duncan) — Gretz, p. 103–104, Pl. 9.
v non 2018 Phacelostylophyllum ?suttonensis (Duncan) — Boivin et al., fig. 3–e-g.
v. 2019 Phacelostylophyllum suttonensis (Duncan) — Boivin, p. 250–251, fig. 10.29.
Remarks. Phacelophyllia suttonensis (Duncan) in Kiessling et al. (2009) is interpreted as a heterogenous assemblage of Heterastraea (Fig. 9.A–C) and a true Phacelophyllia (Fig. 7.F); the latter considered a junior synonym of Thecactinastraea according to Melnikova and Roniewicz (2017). The status of the type specimen of Thecosmilia suttonensis Duncan is still disputable and has been successively attributed to Thecosmilia, Phacelepismilia, Phacelophyllia and Phacelostylophyllum. Obviously, this species cannot remain in its original genus because of the montlivaltiid structure of Thecosmilia. Phacelepismilia has been considered a synonym of Archaeosmiliopsis a genus with compact septa without spines (Kiessling et al., 2009). In addition, the type specimen of the type species (Montlivaltia simplex Duncan) of Phacelepismilia is not clearly phaceloid. The generic attribution of Thecosmilia suttonensis Duncan to Thecactinastraea or Phacelostylophyllum is more difficult, especially because of the unavailability of thin sections. Based on the literature (Duncan, 1867; Beauvais, 1976) and in the absence of direct observation of the type specimen, we assigned this species to Phacelostylophyllum because of the apparent weak ornamentation of septa and the probable development of tabuloid dissepiments.
Type designation. Holotype by monotypy. Collection Moore BRLSI 288.
Type locality and horizon. Hettangian, Angulata chronozone, from Sutton Stone, Wales, Great Britain.
Originally included material. Only the holotype.
Etymology. From sutton referring to the type locality.
Material examined. 1 colony: SB–170 and 3 uncertain silicified colonies: SB–39, SB–87 and SB–192.
Ages and localities of material examined. ?Hettangian–Sinemurian from Dromedary biostrome and upper Sinemurian or lowermost Pliensbachian from Castle olistolith, Amellagou Region, High Atlas Mountains, Morocco.
Description. Colonies phaceloid. Transverse section of branches sub-circular. Radial elements are septa, costae not observed, sub-compact, free, straight. Septal apparatus organised in two or three size orders hierarchized in length. S1 septa are long, reach the axial region and show projections inducing a weak papillose columella. Structure of septa perhaps stylophyllid, difficult to confirm due to the preservation. Endotheca made of large vesicular to tabular dissepiments (Fig. 31B). Wall nature unknown.
Dimensions. Calicular diameter 2.1 to 7.5 mm — Septal density 4 septa per 2 mm.
Similarities and differences. Phacelostylophyllum suttonensis (Duncan, 1867) differs from Phacelostylophyllum martini (Fromentel, 1860) which has larger calices (between 10 and 20 mm diameter). Phacelostylophyllum suttonensis (Duncan, 1867) is very close of Phacelostylophyllum michelini (Terquem and Piette, 1865) but the latter does not show detachments in the axial region.
Stratigraphic and geographic distribution. Hettangian from Wales, Sinemurian from France, Sinemurian − early Pliensbachian from Morocco (High Atlas Mountains).
Genus Pinacophyllum (Frech, 1890)
Type species. Pinacophyllum paralellum (Frech, 1890) by monotypy (ICZN art. 68.3 as Pinacophyllum (?) annulatum (Reuss, 1855) was assigned with doubt to the genus Pinacophyllum by (Frech, 1890): p. 87).
Originally included species. P, paralellum (Frech, 1890), and P. ? annulatum (Reuss, 1855) with doubt.
Similarities and differences. This genus differs from Phacelostylophyllum (Melnikova, 1972) that has vesicular dissepiments and septal spines. Pinacophyllum differs from other phaceloid genera by its prominent character of complete tabulae.
Etymology. From pinaco: table in ancient Greek referring to the tabulae and phyllum: leaf in ancient Greek, a common ending in coral generic names.
Status. Available and valid.
Pinacophyllum sp.
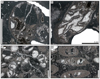 |
Fig. 32 Pinacophyllum sp. A Transverse thin section of the specimen SB–78/2. B Oblique thin section of the specimen SB–78/2. C Transverse thin section of the specimen SB–60. D Oblique thin section of the specimen SB–60. |
v. 2019 Pinacophyllum? sp. — Boivin, p. 252, fig. 10.30.
Material examined. 2 badly preserved colonies: SB–60 and SB–78/2.
Ages and localities of material. Upper Sinemurian from Serdrar reef and upper Sinemurian or lowermost Pliensbachian from Castle olistolith, Amellagou Region, High Atlas Mountains, Morocco.
Description. Phaceloid colonies. Septa compact, short, curved, in small number. Inner margin of septa is occasionally hook-shaped. Endotheca made of large tabuloid dissepiments and complete tabulae. Thick wall of unknown nature.
Dimensions. Diameter of branches 5 to 7 mm — Number of septa circa 6 septa — Thickness of the wall circa 0.5 mm.
Remarks. The very bad preservation made the identification very difficult. However, due to the original character of the endotheca another generic attribution is not considered plausible.
Similarities and differences. We have investigated the nominal species assigned to Pinacophyllum. Among them Pinacophyllum conglomeratum (Frech, 1889) is not an available name (ICZN art. 1.3.5). It was published as a nomen nudum as a species of Amplexus (?). One year later, Frech (1890) stated that the single sample of Amplexus (?) conglomeratus should be temporarily named Pinacophyllum sp. nov. All the other nominal species, such as P. annulatum (Reuss, 1855), P. gracile (Münster, 1841), P. lejowae (Roniewicz, 1974), P. parviseptum (Squires, 1956), P. parallelum (Frech, 1890), P. peruvianum (Stanley, 1994), P. spizzensis (Tornquist, 1899), and P. yunnanense (Wu, 1977), whether or not they belong to the genus Pinacophyllum, have many more septa than our specimen.
Stratigraphic and geographic distribution. Upper Sinemurian — lower Pliensbachian from Morocco (High Atlas Mountains).
Genus Stylophyllopsis (Frech, 1890)
Type species. Stylophyllopsis polyactis (Frech, 1890) by subsequent designation (Diener, 1921).
Originally included species. Stylophyllopsis polyactis (Frech, 1890), Stylophyllopsis zitteli (Frech, 1890), Stylophyllopsis rudis (Emmrich, 1853), Stylophyllopsis caespitosa (Frech, 1890), Stylophyllopsis mojsvari (Frech, 1890), and Stylophyllopsis lindstroemi (Frech, 1890).
Similarities and differences. Stylophyllopsis differs from other solitary genera by its septal structure characterised by septal spines deriving from a microstructure dominated by thickening deposits;
- Coryphyllia (Cuif, 1975a) presents a mid-septal line and generally an elongated fossa.
- Distichophyllia (Cuif, 1975c) is characterised by a mid-septal line in zigzag, which is expressed in the morphology of thin septa.
- Epismiliopsis (Alloiteau, 1952) is very close to Stylophyllopsis, even possibly congeneric, but differs by the elongated fossa and the torsion of lateral septa.
- Neorylstonia (Vasseur et al., 2019) has also poorly ornamented septa but a highly distinctive axial structure.
- Archaeosmiliid genera like Archaeosmilia (Melnikova, 1975a) or Icaunhelia (Beauvais, 1958) have strongly cuneiform septa and no septal spine.
- Proleptophyllia (Alloiteau, 1952) has highly ornamented septa with a dentate distal edge, and a narrow inter-septal space.
Etymology. From stylos: pillar and phyllos: leaf, and opsis: appearance of, in ancient Greek probably referring to the septal spines.
Status. Available and valid.
Stylophyllopsis cf. walliae (Duncan, 1867)
 |
Fig. 33 –Stylophyllopsis cf. walliae (Duncan, 1867). Transverse section of specimen SB–207. |
* 1867 Montlivaltia walliae sp. nov. — Duncan, p. 7, Pl. 8 figs. 5–7.
1867 Thecosmilia plana sp. nov. — Duncan, p. 17, Pl. 3 figs. 24–25.
1868 Montlivaltia rugosa sp. nov. — Duncan, p. 58, Pl. 14 figs. 1–3, Pl. 15 figs. 14, 16 and 17, Pl. 16 figs. 5–15.
1878 Montlivaltia rugosa "Wright" — Tomes, p. 187.
1884b Montlivaltia walliae Duncan — Tomes, p. 363.
1925 Stylophyllopsis rugosa (Duncan & Wright) — Straw, p. 351, fig. 1.1–1.5.
1936 Montlivaltia rugosa "Wright in Duncan" — Joly, p. 169.
1943 Oppelismilia rugosa (Duncan & Wright) — Vaughan & Wells, p. 155, fig. 32a.
1956 Oppelismilia rugosa (Duncan & Wright) — Wells, p. F395, fig. 290.1c.
1975 Stylophyllopsis walliae (Duncan) — Beauvais, p. 114, fig. 3.
1975 Stylophyllopsis rugosa (Duncan) — Beauvais, p. 114, fig. 4.
1975 Stylophyllopsis walliae (Duncan) — Turnšek, Seyfried, & Geyer, p. 135, Pl. 16 figs. 1–2.
1976 Stylophyllopsis walliae (Duncan) — Beauvais, p. 51, Pl. 8 figs 9–10, Pl. 10 figs. 6–7, Pl. 11 figs. 1–2, text-fig. 4–6.
1976 Stylophyllopsis rugosa (Duncan & Wright) — Beauvais, p. 54, pl. 11 fig. 6, text-fig. 10.
1986 Stylophyllopsis walliae (Duncan) — Beauvais, p. 6.
1991 Stylophyllopsis walliae (Duncan) — Negus p. 255 Table 1
1993 Stylophyllopsis rugosa (Duncan & Wright) — Roniewicz & Morycowa, fig. 2.7.
2001 Stylophyllopsis rugosa (Duncan) — Stolarski & Roniewicz, fig. 5.1–5.2.
cf 2002 Stylophyllopsis sp. cf. S. rugosa (Duncan & Wright) — Stolarski & Russo, p. 656, figs. 7–8.
v cf 2019 Stylophyllopsis cf. walliae (Duncan) — Boivin, p. 260, fig. 10.34.
Type designation. Holotype by monotypy, BRLSI n° 260 (collection Moore).
Type locality and horizon. Hettangian, Angulata chronozone, Brocastle deposits, Bridgend, South Wales, Great Britain.
Originally included material. Only the holotype.
Etymology. Hypothetically dedicated to Mrs. Wall (Duncan previously dedicated also a species to Mr. Wall: Antillia walli Duncan and Wall, 1865).
Material examined. 1 specimen: SB–207.
Ages and localities of material. Upper Sinemurian from Serdrar reef, High Atlas Mountains, Morocco.
Description. Solitary coral, corallite circular or elliptical. Septa with stylophyllid structure, subcompact, free or sometimes joined, straight and thick. Septal apparatus organised in two size orders (perhaps three). Inner margin of septa characterised by dissociated free septal spines, which are projected in the lumen and produce a parietal papillose columella. Endotheca made of large dissepiments.
Dimensions. Calicular diameter circa 15–20 mm — Septal density 4 septa per 5 mm — Number of septa circa 46 septa.
Similarities and differences. The bad preservation of our specimen does not permit a firm identification. However, compared to the other nominal species of the genus Stylophyllopsis (Tab. 7), our specimen seems to be close to Stylophyllopsis walliae by its calicular diameter and its number of septa. Table 7 and Figure 34 suggest that the genus has been unreasonably split. We have, however, not the necessary population to solve this issue.
 |
Fig. 34 Plots of dimensions of the type material of Stylophyllopsis nominal species presented in Table 7. |
Stratigraphic and geographic distribution. Hettangian–Sinemurian from Belgium and Morocco, Hettangian from Great Britain, Sinemurian from Great Britain and Sicily, upper Sinemurian from Morocco, upper Sinemurian − Lower Pliensbachian from Spain.
Family Thecosmiliidae (Duncan, 1884)
Genus Vallimeandropsis (Beauvais, 1966a)
Type species. Latomeandra davidsoni (Edwards and Haime, 1851)a by original designation, p. 873.
Originally included species. Only the type species.
Similarities and differences. This genus differs from Microphyllia (Orbigny, 1849) that shows a pennular structure whereas septa of Vallimeandropsis display a montlivaltiid structure.
Etymology. From vallis: valley in Latin, Maiandros: Maeandros, a river of Anatolia famous for its meanders and opsis: appearance of, in ancient Greek referring to its meandroid morphology.
Status. Available and valid.
Vallimeandropsis davidsoni (Edwards and Haime, 1851a)
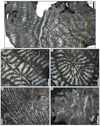 |
Fig. 35 Vallimeandropsis davidsoni (Edwards & Haime, 1851a) A Transverse thin section of the colony SB–335. B–C Enlargements of A. D Longitudinal thin section of the colony SB–143. E Transverse thin section of the colony SB–143. |
* 1851a Latomeandra davidsoni sp. nov. — Edwards & Haime, p. 137, pl. 27 figs. 10, 10a.
1857 Latimæandra davidsoni Edwards & Haime − Edwards & Haime, p. 549.
1861 Latimæandra davidsoni Edwards & Haime — Fromentel, p. 161.
? 1865 Latomaeandra davidsoni Edwards & Haime — Eichwald, p. 145.
1882 Latimæandra davidsoni Edwards & Haime — Tomes, p. 426.
non 1885 Latimæandra davidsoni Edwards & Haime — Koby, p. 248, Pl. 73 fig. 2.
non? 1885 Latimæandra curtata (Etallon) — Koby, p. 223, Pl. 69 figs. 1–3.
1888 Latimaeandra davidsoni Edwards & Haime — Schlippe, p. 83.
? 1888 Latimaeandra davidsoni Edwards & Haime — Meyer, p. 35, Pl. 4 fig. 1.
1890 Latimæandra davidsoni Edwards & Haime — Tomes, p. 304.
non? 1905 Latimaeandra curtata (Etallon) — Koby, p. 105, Pl. 16 fig. 2.
? 1907 Latimæandra davidsoni Edwards & Haime — Koby, p. 31.
? 1955a Microphyllia davidsoni (Edwards & Haime) — Geyer, p. 346.
? 1955b Microphyllia davidsoni (Edwards & Haime) — Geyer, p. 205.
1957 Brachyseris davidsoni (Edwards & Haime) — Alloiteau, p. 313.
1957 Microphyllia davidsoni (Edwards & Haime) — Frajová, p. 52.
non 1958 Microphyllia cf. davidsoni (Edwards & Haime) — Alloiteau, p. 92, Pl. 29 figs. 4, 5, 6.
non? 1958 Isastraea davidsoni (Edwards & Haime) — Alloiteau, p. 44, Pl. 5 fig. 6, Pl. 16 fig. 1; Pl. 19 fig. 5, Pl. 23 fig. 5.
non 1964 Microphyllia curtata? (Etallon) — Beauvais, p. 250, Pl. 35 fig. 1.
v 1966a Vallimeandropsis davidsoni (Edwards & Haime) — Beauvais, p. 873, Pl. 36 fig. 1, Pl. 37 fig. 3.
1970 Vallimæandropsis davidsoni (Edwards & Haime) — Beauvais, p. 61.
1971 Vallimaeandropsis davidsoni (Edwards & Haime) — Beauvais, p. 2.
? 1980 Brachyseris davidsoni (Edwards & Haime) — Ljuljeva & Permjakov, p. 95, Pl. 20 figs. 7, 8.
1986 Microphyllia lineata sp. nov. — Beauvais, p. 58, fig. 2, Pl. 14 fig. 2.
1993 Vallimeandropsis davidsoni (Edwards & Haime) — Pandey & Fürsich, p. 24, Pl. 11 fig. 1.
v. 2010 Vallimeandropsis davidsoni (Edwards & Haime) — Lathuilière, www.corallosphere.org with figures.
v. 2011 Microphyllia? lineata Beauvais — Lathuilière, p. 540, Pl. III figs.1–3.
? 2015 Vallimeandropsis davidsoni (Edwards & Haime) — El-Sorogy & Al-Kahtany, p. 100, fig. 5.H–I.
v. 2018 Vallimeandropsis lineata (Beauvais) — Vasseur, p. 370-371, fig. 3.107.
v. 2019 Vallimeandropsis lineata (Beauvais) — Boivin, p. 262, figs. 10.35–10.36.
Remarks. After an assignation to the genus Brachyseris in 1957, Alloiteau (1958) has simultaneously attributed the species Latomeandra davidsoni (Edwards & Haime, 1851)a to Isastraea (p. 44) and Microphyllia (p. 92). These two names, however, correspond to different taxa: Microphyllia cf. davidsoni (Edwards and Haime, 1851a) in Alloiteau (1958) is a badly preserved colony, which can be possibly referred to Microphyllia, when Isastraea davidsoni (Edwards & Haime, 1851a) in Alloiteau (1958) shows a good thin transverse section (Pl. 16 fig. 1) in which the montlivaltiid microstructure is clearly demonstrated. We attribute this section figured in Alloiteau (1958) to the cerioid genus Isastrea (Edwards and Haime, 1851b). It has been considered as a new species by Beauvais (1966a): Vallimeandropsis vongohensis. The homogeneity of the syntypes of the latter species is not demonstrated.
Latimæandra davidsoni Edwards & Haime in (Koby, 1885) was also erected as a new species: Microphyllia pulchella (Beauvais, 1966b).
Type designation. Neotype, designated by Beauvais, 1966a: p. 873 Pl. 36 fig. 1 and Pl. 37 fig. 3, NHMUK R 21152, also photographed in Corallosphere (Lathuilière, 2010).
Type locality and horizon. Bajocian of Crickley (near Cheltenham, Great Britain).
Originally included material. Only the lost holotype.
Etymology. Dedicated to Thomas Davidson, a famous Scottish palaeontologist.
Material examined. 3 colonies: SB–143, SB–314 and SB–335.
Ages and localities of material. Upper Sinemurian from Serdrar reef and upper Sinemurian or lowermost Pliensbachian from Castle olistolith, Amellagou Region, High Atlas Mountains, Morocco.
Description. Meandroid colonies. Intracalicular budding producing corallites in a single row within a valley and united by a lamellar linkage (Fig. 35E). Corallites distributed in sub-parallel series often curved or slightly sinuous. Collines tectiform without ambulacrum. Septa sub-confluent or confluent on both sides of collines, joined, or sometimes free, straight or curved, sub-compact. Montlivaltiid septal structure with lateral faces of septa granulated. No pennulae. Microstructure trabecular and slight trabecular detachment at the inner margin of septa projected in the axial region. Columella parietal. Septal density highly variable inside valleys. Endotheca made of dissepiments including vesicular dissepiments.
Dimensions. Valley width 2.5 to 5 mm — Colline width 0.3 to 0.7 mm — Septal density 4 to 8 septa per 2 mm — Distance between trabecular axes 120 to 280 µm.
Stratigraphic and geographic distribution. Upper Sinemurian − Lowermost Pliensbachian from Morocco, Middle and upper Toarcian from Morocco, Bajocian from England, Germany?, Bathonian from France? and India, Upper Jurassic from Crimea?, Portugal? and Saudi Arabia?, Tithonian-Berriasian from Czech Republic?
Family Incertae Sedis
Genus indet.
Similarities and differences. This genus differs from other cerioid genera:
— Rhaetiastraea Roniewicz, 1974 that has a papillose or parietal columella, the size orders of the septal apparatus better ordered in length and thickness; Rhaetiastraea as many other actinastreid genera has often S3 in contratingent junction;
— Bussonastrea Beauvais, 1965 that has a characteristic septal budding;
— Edwardsastraea (Roniewicz, 1970) that has a papillose columella;
— Enallocoenia (Orbigny, 1849) that has an extracalicular budding and straight septa;
— Pseudodiplocoenia (Alloiteau, 1958) that has joined septa and a columella of septal origin;
— Sakalavastraea (Alloiteau, 1958) that has a parietal columella made of the junction of major septa;
— Septastraeopsis (Alloiteau, 1953) that has a septal budding and septa joined to columella;
— Toechastrea (Volz, 1896) that has trabecular axes perpendicular to the medioseptal plan in the peripheral part and that tend to alternate and grow obliquely inward near the columella;
— Tropiastrea (Cuif, 1967) with its pennular structure characterised by meniana directed upward.
Gen indet. sp.
 |
Fig. 36 Gen. indet. sp. A transverse section of specimen SB–210/2. B–C Enlargement of A. |
non 1976 Toechastraea pachyphylla sp. nov. — Cuif, p. 146, Pl. 16 figs. 3–4.
? 1987 Toechastraea pachyphyllia Cuif — Turnšek & Ramovš, p. 41–42, Pl. 3 figs. 5–6.
? 1997 Toechastraea pachyphyllia Cuif — Turnšek, p. 207.
v 2019 Gen indet. sp. — Boivin, p. 208, fig. 10.13.
Material examined. 1 colony: SB–210/2.
Ages and localities of material. Upper Sinemurian from Serdrar reef, Amellagou Region, High Atlas Mountains, Morocco.
Description. Colony cerioid. Probably growing by intracalicular budding. Corallite polygonal or elliptical. Septa compact except the inner margin, free, uncommonly joined, confluent or not confluent, curved. Septal apparatus organised in three size orders, not always easy to distinguish (particularly between S1 and S2). Lateral margin of septa ornamented by rounded granules or slightly pointed ones (Fig. 36B). Trabeculae are dissociated at inner margin of septa. Well delimited styliform columella. No pali. Endotheca not visible. Septothecal wall in zigzag.
Dimensions. Calicular great diameter 2.58 to 3.13 mm — Calicular small diameter 2.36 to 2.56 mm — Thickness of the wall 0.22 to 0.25 mm — Distance from corallite to corallite 2.19 to 2.33 mm —Septal density 5 to 6 septa per 1 mm — Trabecular density 3 to 4 per 0.5 mm.
Remarks. Our specimen is very close to the material figured by Turnšek & Ramovš (1987) (also figured in Turnšek, 1997) under the species name Toechastraea pachyphyllia. However, the Slovenian specimen, like ours, does not allow the observation of the typical ornamentation of lateral faces of septa and in particular the typical granules, well preserved of the type material of Toechastraea pachyphyllia (Cuif, 1976). In both cases also, the interseptal space is much wider than in Toechastraea pachyphyllia and septal junctions are not observed.
Stratigraphic and geographic distribution. Norian from Slovenia?, Sinemurian from Morocco (High Atlas Mountains).
Genus Neorylstonia (Vasseur et al., 2019)
nomen novum pro Mesophyllum (Beauvais, 1986) non (Schlüter, 1889)
Type species. Mesophyllum pseudocolumellatum (Beauvais, 1986) by original designation.
Originally included species. Only the type species.
Similarities and differences. This genus differs from other solitary genera by its very characteristic axial structure: the pseudocolumella (see Vasseur et al., 2019 for a precise definition).
Etymology. From neo: new in ancient Greek, and Rylstonia a very similar Palaeozoic rugosan genus.
Status. Available and valid.
Neorylstonia pseudocolumellata (Beauvais, 1986)
 |
Fig. 37 Neorylstonia pseudocolumellata (Beauvais, 1986) A Natural transverse section of the specimen SB–166. B Natural transverse section of the specimen SB–236. C–D Transverse thin section of the specimen SB–236. Section C is more distal (12 mm) than section D. |
*v 1986 Mesophyllum pseudocolumellatum sp. nov. — Beauvais, p. 20, Pl. 4, fig. 1a–1b.
v. 2018 Neorylstonia pseudocolumellata (Beauvais) — Vasseur, p. 259-260, fig. 3.63.
v. 2019 Neorylstonia pseudocolumellata (Beauvais) — Vasseur et al., p. 12–17, fig. 2–6.
v. 2019 Neorylstonia pseudocolumellata (Beauvais) — Boivin, p. 224–225, fig. 10.20.
v. 2021 Neorylstonia pseudocolumellata (Beauvais) — Vasseur & Lathuilière, p. 1234, fig. 32.
Type designation. Holotype by original designation of Beauvais (1986: p. 20, pl. 4 fig. 1a,b) MNHN.F.R11604, Menchikoff collection.
Type locality and horizon. Lower Jurassic from Beni Tadjit, High Atlas Mountains, Morocco.
Originally included material. Four paratypes: MNHN.F.A30500, MNHN.F.R11605 (Pl. 4 fig. 1c–d), MNHN.F.R11606 (Pl. 4 fig. 1e) & MNHN.F.R11607 (Pl. 4 fig. 1f).
Etymology. From pseudocolumella referring to the characteristic axial structure of the genus.
Material examined. 5 specimens: SB–164, SB–166, SB–206, SB–236 and SB–238 and 1 uncertain specimen: SB–241.
Ages and localities of material. Upper Sinemurian from Serdrar reef, upper Sinemurian or lowermost Pliensbachian from Castle olistolith and lower Pliensbachian from Owl olistolith, Amellagou Region, High Atlas Mountains, Morocco.
Description. Solitary coral. Corallum ceratoid. Corallite circular to sub-circular. Costosepta straight or slightly curved, free, compact, occasionally rhopaloid. Radial elements are commonly arranged in three distinct size orders with different length and width in transverse sections but S1 and S2 are occasionally indistinct because of similar sizes. Some first- order septa (S1) rarely reach the axial structure but in most cases the inner edge abruptly dips in the fossa. Lateral faces and distal edge are smooth. No palus. Axial structure present, in the form of a Rugose-like calicular boss consisting of septa and endotheca — a pseudocolumella according to Edwards & Haime (1848) and later to Berkowski & Weyer (2012). This pseudocolumella presents different morphologies during the polyp life, giving the fossils a very characteristic aspect in transverse section. In the main cases, hexameral symmetry can be recognised. The superimposed bilateral symmetry is evidenced by the spatial organization of the axial structure. Endotheca abundant, made of tabular dissepiments regularly arranged in the axial zone. Parathecal wall. Paratheca is continuous with the dissepiments. Synapticulae absent.
Dimensions. Calicular great diameter 14.5 to 20.5 mm — Calicular small diameter 12.5 to 20 mm — Number of septa 40 to 50 septa — Septal density 5 to 7 septa per 1 mm.
Stratigraphic and geographic distribution. Sinemurian − Pliensbachian from Morocco (Middle and High Atlas Mountains), Pliensbachian from Italy.
Comparison of dimensions between species of Lochmaeosmilia and Apocladophyllia.
Dimensions of Lepidophyllia (Heterastraea) microcalix sp. nov. specimens.
Comparisons between dimensions of Lepidophyllia (Heterastraea) nominal species. Two first measurements are in millimetres and rounded to the nearest half-millimetre. D diameter, CC distance from calice to calice, NS number of septa, Sd septal density. The dimensions are those from: 1 Beauvais (1976), 2 Turnšek & Kosir (2000), 3 Turnšek, Buser, & Debeljak (2003), 4 Gretz (2014), 5 Tomes (1888), 6 Terquem & Piette (1865), 7 Fromentel (1860).
Dimensions of type material of Stylophyllopsis nominal species. Diameters are rounded to the nearest half-millimetre. F. Dimensions of figured material in absence of type specimen (S. guettardi: figuration from Lejeune, 1935). H. Dimensions of the holotype. L Dimensions of the lectotype. TS. Dimensions of the type series. S Dimensions of the syntype(s). * Dimensions estimated from figures or photographies. Remark: Stylophyllopsis pontebbanae Volz, 1896 is not included in the table because it has been reassigned to Pontebbastraea Roniewicz & Michalik, 200; 2, a junior synonym of Remismilia Beauvais, 1972 according to Roniewicz, 2010b. Stylophyllopsis laxa (Fromentel & Ferry, 1867) is considered a junior synonym of S. fritillus according to Vasseur & Lathuilière (2021). S. rugosa (Duncan, 1868) is regarded as a junior synonym of S. walliae (Duncan, 1867) according to Beauvais (1986).
4 Results & conclusion
The distribution of the different specimens given in Table 3 was considered in order to assess the representativeness of our samples, from the 4 localities studies, i.e., the Dromedary biostrome, the Serdrar reef, the Castle olistolith and the Owl olistolith. The rarefaction curves (Fig. 38) of the three main localities (Serdrar, Castle and Owl reefs) show a similar relationship between the specific richness and the number of specimens. The Serdrar reef, despite its large size, also compared to the others, is a little different and, according to the global curve, is approaching the maximum number of taxa more quickly. The Dromedary biostrome, the most ancient coral-bearing outcrop, due to its small size, is not representative of the real diversity of the corresponding time.
 |
Fig. 38 Rarefaction curves of the taxa from the different studied localities (i.e., the Dromedary biostrome, the Serdrar reef, the Castle olistolith and the Owl olistolith), in comparison to the global curve that takes into account the whole set of assemblages described in the present study. |
Figure 39A illustrates the distribution of the studied specimens according to their colonial arrangement. It demonstrates not only the important proportion of cerioid and solitary corals but also the occurrence of significant proportions of thamnasterioid and meandroid forms. In term of species, as shown in Figure 39B, the proportion of cerioid species is lower than expected from the Figure 39A. By contrast, the proportion of phaceloid species is significantly higher when compared to the proportion of phaceloid ones. The high proportion of solitary forms remains strong for species and individuals.
 |
Fig. 39 A Distribution of collected specimens from the different studied localities (i.e., the Dromedary biostrome, the Serdrar reef, the Castle olistolith and the Owl olistolith), according to their colonial arrangement. B Distribution of collected species from the different studied localities according to their colonial arrangement. |
The proportion of colonial arrangements among localities, is illustrated in Figure 40A. It can also be viewed in terms of chronology, from the oldest Dromedary biostrome (?Hettangian-Sinemurian) to the youngest Owl olistolith (lower Pliensbachian). It is important to note that Serdrar reef (upper Sinemurian) and Castle olistolith (upper Sinemurian or lowermost Pliensbachian) could be synchronous or even age-reversed. However, as already mentioned, the Dromedary biostrome cannot be reliably interpreted due to its low sampling. The Serdrar reef shows a high proportion of cerioid specimens while the proportion of solitary specimens is rather low. Contrastingly, the Owl olistolith exhibits a low proportion of cerioid specimens compensated by solitary ones. In some regards, the Castle olistolith presents intermediate values between the Serdrar reef and the Owl olistolith, but with a higher ratio of phaceloid specimens than the other two localities.
 |
Fig. 40 A Distribution of collected specimens according to their colonial arrangement and split by their different studied localities (i.e., the Dromedary biostrome, the Serdrar reef, the Castle olistolith and the Owl olistolith), B Distribution of collected species from the different studied localities, according to their colonial arrangement t and spitted by their different studied localities. |
The Figure 40B complements the above results and provides some details on the specific compositions of the colonial arrangements within the different outcrops. Except for the Dromedary biostrome, because of the low number of samples, the proportions of solitary and cerioid species remain stable between all localities, while the proportion of solitary and cerioid specimens varies (see Fig. 40A). Finally, a great homogeneity is observed between the distributions of the colonial arrangements of Serdrar reef and Castle olistolith. In contrast, the Owl olistolith is characterised by a higher proportion of thamnasterioid species and a lower proportion of phaceloid species.
To summarize, the present study was conducted in the Amellagou Region in Moroccan High Atlas Mountains. More precisely concern the Dromedary biostrome (?Hettangian–Sinemurian), the Serdrar reef (upper Sinemurian), the Castle olistolith (upper Sinemurian or lowermost Pliensbachian) and the Owl olistolith (lower Pliensbachian).
Thanks to this new taxonomic assessment, the last appearance datum of the several genera has been changed, namely Chondrocoenia, Parastraeomorpha, Araiophyllum, Paracuifia, Pinacophyllum, and possibly Paravolzeia. The first appearance datum of Proleptophyllia, Vallimeandropsis, and possibly Lochmaeosmilia has also been revised. These modifications equally apply to several species (cf. Tab. 3). The present study, devoted solely to the lower Liassic of Morocco, fills a gap between Upper Triassic and upper Liassic records. In addition, it completes the recent data of Vasseur & Lathuilière (2021) for the Pliensbachian thanks to the data from the owl Olistolith. This study gives a very important indication that, although the Pliensbachian appears to be the best-represented stage of the Early Jurassic, its fossil record is still far from complete. Some Lazarus taxa known from the Triassic and Pliensbachian remain absent in the fossil record from Hettangian and, to a lesser degree now, from the Sinemurian. Therefore, we assume that the absence of these taxa is only due to the poor preservation of coral environments during these periods. This assumption is reinforced by the fact that several new occurrences correspond to a single specimen.
We now have new data to question the relative severity of the end-Triassic crisis in comparison to the Pliensbachian–Toarcian crisis. In this reassessment, the potential continuity of genera from the Triassic to the Jurassic should be re-evaluated. For instance, is there an evolutionary gap between the Triassic Thamnasteriamorpha and the Jurassic Periseris? Is Chondrocoenia so far from Jurassic actinastreids (e.g., Allocoenia, Allocoeniopsis, Stephanastrea)? At the family level, the structure of the Triassic distichophyllid Reimaniphylliidae should be compared with that of the Jurassic montlivaltid Thecosmiliidae. Could these two structures, up to now considered as assuredly separated, also be considered as the two end-members of an evolutionary continuum? In this regard, the Triassic material described by Deng & Zhang (1984a) from China provides questioning evidences for an early onset of the montlivaltid structure (notably Pl. 2 fig. 1b and 4b, Pl. 3 figs. 1c and 3b).
The new data provides here also new light on the question of the relationship between corals and zooxanthellae in the post-end-Triassic crisis context. In particular, the hypothesis of the loss of symbiosis (Rosen, 2000) can be tested. A significant relationship between colonial structures and photosymbiosis is established (e.g., Coates & Oliver, 1973; Coates & Jackson, 1987). On the above basis, we consider that the occurrence of highly integrated forms, such as thamnasterioid and meandroid colonial structures in our outcrops, is a strong argument for the existence of a photosymbiosis. Presently, we know only one exception to this principle, with the thamnasterioid troglodytic species Leptoseris troglodyta Hoeksema, 2012 which has lost its zooxanthellae (Hoeksema, 2012). A further strong argument for the existence of corals with zooxanthellae as early as the lowermost Jurassic is the existence of two species demonstrating a morphological change from lamellar to ramose that cannot be explained without the photosymbiosis. Indeed, Hispaniastraea murciana and Chondrocoenia clavellata show this morphological change that is known in the recent zooxanthellate coral Synaraea convexa (Verrill, 1864) (Jaubert, 1977). However, this does not imply that all corals in this epoch were zooxantellate. It can be considered that a significant proportion of solitary forms were possibly not. The complexity of the evolutionary interactions between different coral genera and zooxanthellae lineages, and their effect on the efficiency of carbonate production remains unknown.
Finally, the investigation of Moroccan Liassic outcrops has significantly improved our knowledge of corals’ evolution. There is still much scope for further explorations to take advantage of the high quality and quantity of Liassic coral-bearing outcrops in Morocco. However, the new knowledge from the Moroccan fossil record needs to be completed by other Liassic localities, both from Tethys and Panthalassa, to reach the global picture of coral evolution during the Liassic.
Acknowledgments
We are thankful to Christophe Durlet for sharing his knowledge on the area, Rowan Martindale, Iuliana Lazar, Raphaël Vasseur, and Khalid El Mhidi (from the Direction de la Géologie, Ministère de l’Energie, des Mines et du Développement Durable − Département de l’Energie et des Mines, Rabat, Morocco) for their valuable help in the field. François Gischig is appreciated for the great quality of the thin sections used in this study. The presented research has been fully financed by the Swiss National Science Foundation, as a part of the REEFCADE project (Grants 200020_156422 and 200020_178908 by Rossana Martini). We are thankful to the Ousri family for their warm welcome in Amellagou and more specifically Moha Ousri for his help in the field.
Supplementary Material
Supplementary Table 1. List of species with their number by localities (including doubtful assignations) and their colonial structures. This table is a reorganised version of Table 3.
Supplementary Table 2. Measurements of Lepidophyllia (Heterastraea) microcalix sp. nov specimens used for Figure 27 principal component analysis.
Access hereReferences
- Alloiteau J. 1939. Deux espèces nouvelles de polypiers d’anthozoaires de l’Anatolie septentrionale. Bull Sci Bourgogne 9: 5–11. [Google Scholar]
- Alloiteau J. 1952. Embranchement des coelentérés. In: Piveteau J, ed. Traité de paléontologie. Tome premier. Paris: Masson, pp. 376–684. [Google Scholar]
- Alloiteau J. 1953. Sur cinq genres nouveaux de Madréporaires post-paléozoïques. Bull la Société Géologique Fr. 6e série 3: 873–887. [Google Scholar]
- Alloiteau J. 1956. Proleptophyllia granulum. Palaeontol Universalis, N.S. 121: 1–2. [Google Scholar]
- Alloiteau J. 1957. Contribution a la systématique des madréporaires fossiles. Paris: Centre National de la Recherche Scientifique, 462, 20 pl. p. [Google Scholar]
- Alloiteau J. 1958. Monographie des Madréporaires fossiles de Madagascar. Ann Géologiques Madagascar 25: 1–218, pl. 1–38. [Google Scholar]
- Beauvais L. 1958. Une nouvelle forme de polypier dans le Jurassique supérieur de l’Yonne Icaunhelia michelini nov. gen., nov. sp. Bull Soc Géol Fr 8 (6): 621–628. [CrossRef] [Google Scholar]
- Beauvais L. 1964. Étude stratigraphique et paléontologique des formations à madréporaires du Jurassique supérieur du Jura et de l’Est du Bassin de Paris. Mémoires la Société Géologique Fr. 43: 1–287, pl. 1–38. [Google Scholar]
- Beauvais L. 1965. Un nouveau mode de bourgeonnement chez les madréporaires post-paléozoïques. Comptes Rendus des Séances l’Académie des Sci. 260 (1): 247–249. [Google Scholar]
- Beauvais L. 1966a. Révision de quelques madréporaires du Dogger d’Angleterre de la collection Milne Edwards. Bull la Société Géologique Fr. 7e série 7 (7): 871–875. [Google Scholar]
- Beauvais L. 1966b. Révision des madréporaires du Dogger de la collection Koby. Eclogae Geol Helv 59: 989–1024, pl. 1–15. [Google Scholar]
- Beauvais L. 1967. Madréporaires. I. Révision des Madréporaires du Dogger des collections A. d’Orbigny et H. Michelin conservées au Muséum national d’histoire naturelle de Paris. Mémoires la Société Géologique Fr. 106: 1–54, pl. 1–4. [Google Scholar]
- Beauvais L. 1970. Madréporaires du Dogger: études des types de Milne-Edwards et Haime. Ann Paléontologie 56: 39–74. [Google Scholar]
- Beauvais L. 1971. Essai de répartition stratigraphique des madréporaires du Dogger. Comptes Rendus l’Académie des Sci. − Série D 272: 3256–3259. [Google Scholar]
- Beauvais L. 1972. Deux nouveaux genres de Madréporaires triasiques. Bull la Société Géologique Fr. 7e série 14: 310–314, pl. 6. [Google Scholar]
- Beauvais L. 1975. Madréporaires du Jurassique. In: Pomerol C, ed. Stratigraphie et Paléogéographie, Ere mésozoïque. Paris: Doin, pp. 113–114. [Google Scholar]
- Beauvais L. 1976. Madréporaires du Jurassique. Révision des types de madréporaires liasiques décrits par Duncan (1867). Mémoires la Société géologique Fr. NS (55): 43–82. [Google Scholar]
- Beauvais L. 1980. Les Calcarea (Spongiaires) du Lias du Maroc. Ann Paléontologie 66 (1): 21–41. [Google Scholar]
- Beauvais L. 1981. Sur la taxinomie des Madréporaires mésozoïques. Acta Palaeontol Pol 25 (1980) 345–360. [Google Scholar]
- Beauvais L. 1982. Étude de quelques coelentérés de la base du Mésozoïque du Canada occidental. Can J Earth Sci 19 (10): 1963–1973, pl. 1–2. [CrossRef] [Google Scholar]
- Beauvais L. 1986. Monographie des madréporaires du Jurassique inférieur du Maroc. Palaeontogr Abteilung A Paläozoologie, Stratigr 194: 1–68, pl. 1–15. [Google Scholar]
- Beauvais L, Chaix C, Lathuilière B, Löser H. 1993. Termes morphologiques utilisés pour décrire les scléractiniaires: liste préliminaire. Foss Cnidaria Porifera Newsl 22: 50–69. [Google Scholar]
- Bengtson P. 1988. Open nomenclature. Palaeontology 31 (1): 223–227. [Google Scholar]
- Berg HC, Cruz EL. 1982. Map and table describing fossil collections and related samples in the Ketchikan and Prince Rupert Quadrangles, southeastern Alaska. United States Geol Surv Open-File Re p 82–1082 1–26. [Google Scholar]
- Berkowski B, Weyer D. 2012. Hamaraxonia, a new pseudocolumellate genus of Middle Devonian deep-water Rugosa (Anthozoa) from Morocco. Geol Belgica 15 (4): 245–253. [Google Scholar]
- Bernecker M. 1996. Upper Triassic reefs of the Oman Mountains: data from the South Tethyan margin. Facies 34 (1): 41–76. [CrossRef] [Google Scholar]
- Bernecker M. 2005. Late Triassic reefs from the Northwest and South Tethys: distribution, setting, and biotic composition. Facies 51 (1-4): 442–453. [CrossRef] [Google Scholar]
- Bertling M. 1995. Autecological case study of Late Jurassic Thamnasteria (Scleractinia) species with small corallites. In Lathuilière B, Geister J, eds. Coral reefs in the past, present and future. Luxembourg: Service Géologique du Luxembourg, Vol. V 29, pp. 111–117. [Google Scholar]
- Blainville H-MD de. 1830. Zoophytes. In: Levrault FG, ed. Dict des Sci Nat 60: 1–631. [Google Scholar]
- Blodgett RB, Wilson FH, Stanley GDJ, McRoberts CA, Sandy MR. 2000. Upper Triassic stratigraphy and fauna of the Taylor Mountains D-2 and D-3 quadrangles (SW part of the Farewell terrane), southwest Alaska. Geol Soc Am Abstr Programs 32 (6): A–4. [Google Scholar]
- Bo J, Yao J, Liao W, Deng Z-Q. 2017. Triassic scleractinian corals in China: a review of present knowledge. Acta Geol Sin − English Ed 91 (1): 270–282. [CrossRef] [Google Scholar]
- Boivin S. 2019. Coral recovery after the End-Triassic mass extinction: insights on the Liassic biodiversity from Moroccan and French new collections. Doctoral Thesis. Geneva, Switzerland: Université de Genève, 385 p. [Google Scholar]
- Boivin S, Gretz M, Lathuilière B, Olivier N, Bartolini A, Martini R. 2018. Coral- and oyster-microbialite patch reefs in the aftermath of the Triassic-Jurassic biotic crisis (Sinemurian, Southeast France ). Swiss J Geosci. [Google Scholar]
- Boivin S, Vasseur R, Lathuilière B, et al. 2019. A little walk between early Jurassic sponges and corals: a confusing morphological convergence. Geobios 57: 1–24. [CrossRef] [Google Scholar]
- Bourne GC. 1900. The Anthozoa. In: Ray Lankester E, ed. A Treatise on Zoology. The Porifera and Coelentera. London: Adam & Charles Black, pp. 1–84. [Google Scholar]
- Brame H-MR, Martindale RC, Ettinger NP, et al. 2019. Stratigraphic distribution and paleoecological significance of Early Jurassic (Pliensbachian-Toarcian) lithiotid-coral reefal deposits from the Central High Atlas of Morocco. Palaeogeogr Palaeoclimatol Palaeoecol 514: 813–837. [CrossRef] [Google Scholar]
- Buser S, Ramovš A, Turnšek D. 1982. Triassic reefs in Slovenia. Facies 6 (1): 15–23. [CrossRef] [Google Scholar]
- Bychkov YM, Melnikova GK. 1985. First discovery of Late Triassic corals in the Koryak highland (in Russian). Dokl AN SSSR 280: 159–161. [Google Scholar]
- Caruthers AH, Stanley GDJ. 2008. Systematic analysis of Upper Triassic silicified scleractinian corals from Wrangellia and the Alexander terrane, Alaska and British Columbia. J Paleontol 82 (3): 470–491. [Google Scholar]
- Chao A, Gotelli NJ, Hsieh TC, et al. 2014. Rarefaction and extrapolation with Hill numbers: a framework for sampling and estimation in species diversity studies. Ecol Monogr 84 (1): 45–67. [CrossRef] [Google Scholar]
- Clapp CH, Shimer HW. 1911. The Sutton Jurassic of the Vancouver Group, Vancouver Island. Proc Bost Soc Nat Hist 34: 426–438 + Pl. 40–41. [Google Scholar]
- Coates AG, Jackson BC. 1987. Clonal growth, algal symbiosis, and reef formation by corals. Paleobiology 13: 363–378. [Google Scholar]
- Coates AG, Oliver WAJ. 1973. Coloniality in zoantharian corals. In Boardman RS, Cheetham AA, Oliver WAJ, eds. Animal colonies development and function through time. Dowden, Stroudsburg, Pennsylvania: Hutchinson BT & Ross Inc. pp. 3–27. [Google Scholar]
- Cuif J-P. 1966. Structure de quelques polypiers phaceloïdes triasiques. Bull Soc Géol Fr S7-VIII(1): 125–132. [CrossRef] [Google Scholar]
- Cuif J-P. 1967. Note sur le genre Toechastraea Volz 1896. Bull la Société Géologique Fr 7e série 9: 903–908, pl. 33. [Google Scholar]
- Cuif J-P. 1975a. Recherches sur les Madréporaires du Trias. II. Astraeoida. Révision des genres Montlivaltia et Thecosmilia. Étude de quelques types structuraux du Trias de Turquie. Bull du Muséum Natl Hist Nat (Sciences la Terre) 40 /275: 293–400. [Google Scholar]
- Cuif J-P. 1975b. Recherches sur les madréporaires du Trias. III. Etude des structures pennulaires chez les madréporaires triasiques. Bull du Muséum Natl Hist Nat (Sciences la Terre) 44 /310: 45–127. [Google Scholar]
- Cuif J-P. 1975c. Caractères morphologiques, microstructuraux et systématiques des Pachythecalidae nouvelle famille de Madréporaires triasiques. Geobios 8 (3): 157–180. [CrossRef] [Google Scholar]
- Cuif J-P. 1976. Recherches sur les Madréporaires du Trias. IV. formes cério-méandroides et thamnastérioïdes du Trias des Alpes et du Taurus sud-anatolien. Bull du Muséum Natl Hist Nat (Sciences la Terre) 55 /381: 1–191. [Google Scholar]
- Cuif J-P. 1977. Arguments pour une relation phylétique entre les Madréporaires paléozoïques et ceux du Trias. Mémoires la Société Géologique Fr 56: 1–54, pl. 1–13. [Google Scholar]
- Cuif J-P. 1980. Microstructure versus morphology in the skeleton of triassic Scleractinian corals. Acta Paleontol Pol 25: 361–374. [Google Scholar]
- Cuif J-P., Gautret P. 1993. Evolution des Scleractiniaires: diversité des architectures poreuses au Trias supérieur. Geobios 26 (4): 405–412. [CrossRef] [Google Scholar]
- Deng Z-Q. 2006. Middle Triassic corals from W. Guangxi and S. Guizhou. Acta Palaeontol Sin (1): 32–51. [Google Scholar]
- Deng Z-Q., Zhang Y. 1984a. Late Triassic Scleractinian corals from the Hengduan Mountains in Southwest China. In Sichuan W, Xizang E, eds. Stratigraphy and Palaeontology. China. Part 4. Chengdu: Sichuan Science and Technology Publishing House, pp. 203–283. [Google Scholar]
- Deng Z-Q., Zhang Y. 1984b. Supplemental notes on Mesozoic Scleractinia from Mts. Hengduan, southwest China [ in Chinese with English summary]. Bull Nanjing Inst Geol Palaeontol 12: 285–307. [Google Scholar]
- Diener C. 1921. Fossilium catalogus: I. Animalia; Pars.13, Cnidaria triadica 46 p. [Google Scholar]
- Douglas JA. 1929. A Marine Triassic Fauna from Eastern Persia. Q J Geol Soc 85 (1-4): 624–650. [CrossRef] [Google Scholar]
- Dumortier E. 1864. Études paléontologiques sur les dépôts Jurassiques du bassin du Rhône − Première Partie: Infra-Lias. Paris, France: F. Savy éd., Libraire des Sociétés Géologiques et Métérologiques de France, 187 pp. + 30 pl. p. [Google Scholar]
- Duncan PM. 1865 −1868. A monograph of the British fossil corals. Second series. Monogr Palaeontogr Soc. [Google Scholar]
- Duncan PM. 1867. A monograph of the British fossil corals. Second series. Part IV, No. 1. Corals from the zone of Ammonites planorbis and Ammonites angulatus in the Liassic formation. Monogr Palaeontogr Soc 20: 1–43, pl. 1–11. [CrossRef] [Google Scholar]
- Duncan PM. 1868. A monograph of the British fossil corals. Second series. Part IV, No. 2. Corals from the zone of Ammonites angulatus (continued). Corals from the zone of Ammonites bucklandi (bisulcatus), Ammonites obtusus, Sow., and Ammonites raricostatus, Ziet. Monogr Palaeontogr Soc 21: 45–73, pl. 12–17. [CrossRef] [Google Scholar]
- Duncan PM. 1884. A revision of the families and genera of the sclerodermic Zoantharia, Ed. and H., or Madreporaria (M. Rugosa excepted). J Linn Soc London Zool 18 (104-105): 1–204. [CrossRef] [Google Scholar]
- Duncan PM, Wall GP. 1865. A notice of the geology of Jamaica, especially with reference to the district of Clarendon with descriptions of the Cretaceous, Eocene, and Miocene corals of the islands. Q J Geol Soc 21 (1-2): 1–15, pl. 1–2. [CrossRef] [Google Scholar]
- Edwards HM, Haime J. 1848. Observations sur les polypiers de la famille des astréides. C R Hebd Seances Acad Sci 27: 465–469. [Google Scholar]
- Edwards HM, Haime J. 1851a A monograph of the British fossil corals. Second part. Corals from the oolitic formations. Palaeontogr Soc Monogr 5: 73–145, pl. 12–30. [Google Scholar]
- Edwards HM, Haime J. 1851b Monographie des polypiers fossiles des terrains palæozoïques, précédée d’un tableau général de la classification des polypes.Arch du Muséum d’Histoire Nat 5: 1–502. [Google Scholar]
- Edwards HM, Haime J. 1857. Histoire naturelle des coralliaires, ou polypes proprement dits. Tome second. Zoanthaires sclérodermés (Zoantharia Sclerodermata) ou madréporaires. Paris: Roret, Vol. 2, 633 p. [Google Scholar]
- Eichwald E. 1865. Lethaea rossica ou Paléontologie de la Russie, Vol. 2, 113–170 p. [Google Scholar]
- El-Sorogy AS, Al-Kahtany KM. 2015. Contribution to the scleractinian corals of Hanifa Formation, Upper Jurassic, Jabal Al-Abakkayn, central Saudi Arabia. Hist Biol 27 (1): 90–102. [CrossRef] [Google Scholar]
- Emmrich A. 1853. IX. Geognostische Beobachtungen aus dem östlichen bayerischen und den angrenzenden österreichischen Alpen. II. Aus dem Gebiete des Alpenkalkes. Jahrb der Kais Geol Reichsanstalt 4: 326–394. [Google Scholar]
- Ferry H de. 1870. Polypiers nouveaux ou peu connus. Ann l’Académie Mâcon 9: 189–206. [Google Scholar]
- Fischer J-C. 1970. Révision et essai de classification des Chætetida (Cnidaria) post-paléozoïques. Ann Paleontol LVI(2): 149–233. [Google Scholar]
- Flügel E, Kiessling W. 2002. Patterns of Phanerozoic reef crises. In Kiessling W, Flügel E, Golonka J, eds. Phanerozoic reef patterns. SEPM (Society for Sedimentary Geology) Special Publication, pp. 691–733. [CrossRef] [Google Scholar]
- Frajova H. 1957. Nove vysledky vyzkumu koralove fauny ze Stramberka, Skalicky a Jasenice na Morave. Zpravy o Geol Vyzk.: 51–54. [Google Scholar]
- Frech F. 1889. Über die Korallenfaunen der nordalpinen Trias. Jahrb der Geol Reichanstalt 39: 489–496. [Google Scholar]
- Frech F. 1890. Die Korallenfauna der Trias. Palaeontographica 37: 1–116, pl. 1–21. [Google Scholar]
- de Fromentel E. 1860. Polypiers. In: J. Martin, ed., Paléontologie stratigraphique de l’Infralias du département de la Côte d’Or. Mémoires la Société Géologique Fr 2e série 7 (1): 1-100, pl. 1–8. [Google Scholar]
- Fromentel E de. 1861. Introduction à l’étude des Polypiers fossiles. Mémoires la Société d’Émulation du Département du Doubs, 3e série 5: 1–357. [Google Scholar]
- Fromentel E de, Ferry H de. 1865a Zoophytes: terrain jurassique (partie 1). In: Orbigny A d’, ed., Paléontologie Française. Paris: Masson, pp. 1–48, pl. 1–12. [Google Scholar]
- Fromentel E de, Ferry H de. 1865b. Zoophytes: terrain jurassique (partie 2). In: Orbigny A d’, ed., Paléontologie Française. Paris: Masson, Vol. V 8, pp. 49–96, pl. 13–24. [Google Scholar]
- Fromentel E de, Ferry H de. 1866. Zoophytes: terrain jurassique (partie 3). In: Orbigny A d’, ed., Paléontologie Française. Paris: Masson, pp. 97–144, pl. 25–36. [Google Scholar]
- Fromentel E de, Ferry H de. 1867. Zoophytes: terrain jurassique (partie 4). In: Orbigny A d’, ed., Paléontologie Française. Paris: Masson, pp. 145–192, pl. 37–48. [Google Scholar]
- Fromentel E de, Ferry H de. 1869. Zoophytes: terrain jurassique (partie 5). In: Orbigny A d’, ed., Paléontologie Française. Paris: Masson, pp. 193–240, pl. 49–60. [Google Scholar]
- Gerth H. 1926. Anthozoa. In: Jaworski E, ed., La fauna del Lias y Dogger de la Cordillera argentina, Actas de la Acad. Nac. de Cienc. Cordoba. pp. 141–143. [Google Scholar]
- Gerth H. 1928. Beiträge zur kenntnis der Mesozoischen Korallenfaunen von Südamerika. Leidse Geol Meded 3: 3–16. [Google Scholar]
- Geyer OF. 1955a. Korallen Fauna aus dem oberen Jura von Portugal. Senckenbergiana Lethaea 35: 317–356. [Google Scholar]
- Geyer OF. 1955b. Beiträge zur Korallenfauna des Stramberger Tithon. Paläontologische Zeitschrift 29 (3/4): 177–216, pl. 22–26. [CrossRef] [Google Scholar]
- Gregory JW. 1900. The corals. In: The Jurassic fauna of Cutch. Mem Geol Surv India, Palaeontol Indica Ser IX 2 (2): 1–195, pl. 1–26. [Google Scholar]
- Gregory JW. 1930. The fossil corals of Kenya colony collected by Miss McKinnon Wood. Monogr Geol Dep Hunterian Museum Glas Univ 4 (10): 185–209. [Google Scholar]
- Gretz M. 2014. Biotic, environmental and climatic changes across the Triassic-Jurassic boundary: causes and effects highlighted via the Liassic coral reef associations. University of Geneva, 290 p. [Google Scholar]
- Gretz M, Lathuilière B, Martini R. 2014. A new coral with simplified morphology from the oldest known Hettangian (Early Jurassic) reef in southern France. Acta Palaeontol Pol 60 (5): 277–286. [Google Scholar]
- Gretz M, Lathuilière B, Martini R, Bartolini A. 2013. The Hettangian corals of the Isle of Skye (Scotland): an opportunity to better understand the palaeoenvironmental conditions during the aftermath of the Triassic-Jurassic boundary crisis. Palaeogeogr Palaeoclimatol Palaeoecol 376(C): 132–148. [CrossRef] [Google Scholar]
- Hahn F. 1911. Neue Funde in nordalpinen Lias der Achenseagegend und bei Ehrwald. Neues Jahrb für Mineral Geol und Paläontologie, Beilage Band 32: 537–557. [Google Scholar]
- Hammer Ø, Harper DAT, Ryan PD. 2001. Past: paleontological statistics software package for education and data analysis. Palaeontol Electron 4 (1). [Google Scholar]
- Hodges MS, Stanley GDJ. 2015. North American coral recovery after the end-Triassic mass extinction, New York Canyon, Nevada, USA. GSA Today 15 (10): 4–9. [CrossRef] [Google Scholar]
- Hoeksema BW. 2012. Forever in the dark: the cave-dwelling azooxanthellate reef coral Leptoseris troglodyta sp. n. (Scleractinia, Agariciidae). Zookeys 228: 21–37. [CrossRef] [Google Scholar]
- ICZN. 1999. International code of zoological nomenclature, 4th ed. London: International Trust for Zoological Nomenclature, 306 p. [Google Scholar]
- Iljina T, Melnikova GK. 1986. Corals used as indicators for stratigraphy of carbonate. In: Parastratigraficheskie gruppy flory i fauny traisa [Parastratigraphic groups of the Triassic fauna and flora], pp. 30-67. [Google Scholar]
- Jaubert J. 1977. Light, metabolism and growth forms of the hermatypic scleractinian coral Synaraea convexa (Verrill) in the lagoon of Moorea (French Polynesia). Vol. Miami, p. 4. Proc 3rd intern. coral reef symp. [Google Scholar]
- Joly H. 1936. Les fossiles du Jurassique de la Belgique avec description stratigraphique de chaque étage. Deuxième partie: Lias inférieur. Bruxelles, Belgique: Musée Royal d’Histoire Naturelle De Belgique. (Mémoire du Musée Royal d’Histoire Naturelle de Belgique). [Google Scholar]
- Kanmera K, Furukawa H. 1964. Triassic Coral Faunas from the Konosé Group in Kyushu: With Notes on Stratigraphy. Mem Fac Sci Kyushu Univ D Geol Kyushu 15 (1): 117–147. [Google Scholar]
- Kiessling W, Aberhan M, Brenneis B, Wagner PJ. 2007. Extinction trajectories of benthic organisms across the Triassic-Jurassic boundary. Palaeogeogr Palaeoclimatol Palaeoecol 244: 201. [CrossRef] [Google Scholar]
- Kiessling W, Roniewicz E, Villier L, Léonide P, Struck U. 2009. An early Hettangian coral reef in southern France: implications for the end-Triassic reef crisis. Palaios 24 (10): 657–671. [CrossRef] [Google Scholar]
- Kittl E. 1903. Geologie der Umgebung von Sarajevo. Jahrb der [Kaiserlich-Königlichen] Geol Reichanstalt 53: 515–748. [Google Scholar]
- Koby F. 1884. Monographie des polypiers jurassiques de la Suisse. Troisième partie. Mémoires la Société Paléontologique Suisse 10: 109–148, pl. 31–42. [Google Scholar]
- Koby F. 1885. Monographie des polypiers jurassiques de la Suisse (5). Mémoires la Société Paléontologique Suisse 12: 213–304, pl. 64–89. [Google Scholar]
- Koby F. 1889. Monographie des polypiers jurassiques de la Suisse (9). Mémoires la Société Paléontologique Suisse 16: 457–586, pl. 121–130. [Google Scholar]
- Koby F. 1894. Monographie des polypiers jurassiques de la Suisse. Deuxième supplément. Mémoires la Société Paléontologique Suisse 21: 1–20. [Google Scholar]
- Koby F. 1905. Description de la faune jurassique du Portugal, polypiers du Jurassique supérieur. Commun da Commissao do Serv Geol Port.: 1–167. [Google Scholar]
- Koby F. 1907. Polypiers bathoniens de St Gaultier. Mémoires la Société Paléontologique Suisse 33: 1–61. [Google Scholar]
- Kolosváry G. 1966a. Angabe zur Kenntnis der Triaskorallen und der begleitenden fauna der CSSR. (V). Geol Pr 38: 179–188. [Google Scholar]
- Kolosváry G. 1966b. Über Triaskorallenfauna Ungarns. Acta Biol 12: 125–138. [Google Scholar]
- Krencker FN, Bodin S, Hoffmann R, et al. 2014. The middle Toarcian cold snap: Trigger of mass extinction and carbonate factory demise. Glob Planet Change 117: 64–78. [Google Scholar]
- Kristan-Tollmann E, Tollmann A, Hamedani A. 1980. Beiträge zur Kenntnis der Trias von Persien. II. Zur Rhätfauna von Bagerabad bei Isfahan (Korallen). Mitteilungen der Osterr Geol Gesellschaft 73: 163–235. [Google Scholar]
- Lachkar N, Guiraud M, El Harfi A, Dommergues J-L., Dera G, Durlet C. 2009. Early Jurassic normal faulting in a carbonate extensional basin: characterization of tecton- ically driven platform drowning (High Atlas rift, Morocco). J Geol Soc 166 (3): 413–430. [CrossRef] [Google Scholar]
- Lamouroux JUF. 1821. Exposition méthodique des genres de l’ordre des polypiers. Paris: Agasse, 115, 84 pl. p. [Google Scholar]
- Lathuilière B. 1990. Periseris, scléractiniaire colonial jurassique. Révision structurale et taxinomie de populations bajociennes de l’est de la France. Geobios 23: 33–55, pl. 1–6. [CrossRef] [Google Scholar]
- Lathuilière B. 1996. Itinéraires astogéniques chez des coraux simples et coloniaux montlivaltiides du Bajocien de France. Geobios 29 (5): 577–603. [CrossRef] [Google Scholar]
- Lathuilière B. 2000. Coraux constructeurs du Bajocien inférieur de France: 2ème partie. Geobios 33 (2): 153–181. [CrossRef] [Google Scholar]
- Lathuilière B. 2010. Corallosphere: Vallimeandropsis. Available from http://www.corallosphere.org/taxon/1586.html (last consult: 21/04/2021) [Google Scholar]
- Lathuilière B. 2011. Faune corallienne des récifs toarciens du moyen Atlas marocain première approche. Bull Soc Géol Fr 182: 533–544. [CrossRef] [Google Scholar]
- Lathuilière B. 2013. Corallosphere: Stylastraea. Available from http://www.corallosphere.org/taxon/1448.html (last consult: 14/01/2023) [Google Scholar]
- Lathuilière B, Marchal D. 2009. Extinction, survival and recovery of corals from the Triassic to Middle Jurassic time. Terra Nov 21 (1): 57–66. [CrossRef] [Google Scholar]
- Lathuilière B, Schlögl J, Tomašových A, Ivanova DK. 2023. Coral assemblages and environments from Bajocian reefs in the Western Carpathians. Geobios 79: 17–41. [CrossRef] [Google Scholar]
- Le Maître D. 1935. Spongiomorphides et algues. Description des spongiomorphides et des algues. Notes Mémoires du Serv Géologique du Maroc 34: 18–58. [Google Scholar]
- Le Maître D. 1937. Nouvelles recherches sur les spongiomorphides et les algues du Lias et de l’Oolithe inférieure. Notes Mémoires du Serv Géologique du Maroc 43: 1–25. [Google Scholar]
- Le Sauvage M. 1823. Mémoire sur un genre nouveau de polypier fossile. Mémoires la Société d’Histoire Nat Paris 1: 241–244. [Google Scholar]
- Lejeune M. 1935. Montlivaultia. Contribution à l’étude biologique des hexacoralliaires fossiles. Ann Paléontologie 24: 99–135. [Google Scholar]
- Liao W. 1982. Mesozoic scleractinia corals from Xizang (Tibet). Ser Sci Exped Qinghai-Xizang Plateau 4: 151–183. [Google Scholar]
- Liao W, Deng Z. 2013. Mesozoic Scleractinian corals of China. Hefei: University of Science and Technology of China Press, 222 p. [Google Scholar]
- Liao W, Li Z. 1979. Paleontological atlas of the northwest region, Shanghai. Sub-volume (II), The subclass Hexacoralla Coral. Geological Publications. [Google Scholar]
- Liao W, Xia J. 1993. Mesozoic and Early Cenozoic scleractinian corals from Tibet. Cour Forschungsinstitut Senckenb 164: 205–210. [Google Scholar]
- Liao W, Xia J. 1994. Mesozoic and Cenozoic scleractinian corals from Xizang [in Chinese with English summary]. Palaeontol Sin Ser B 184: 1–252. [Google Scholar]
- Lissajous M. 1912. Jurassique maconnais. Description des fossiles caractéristiques et des espèces les plus communes. Bull Soc Hist Nat Macon 3 (16): 177—208. [Google Scholar]
- Ljuljeva SA, Permjakov VV. 1980. Kokkolitoforidy i korraly mezozoja ukranij. Paleontologitcheskij spravotchink., Akademiya Nauk Ukrainskoj SSR Institut Geologitcheckikh Nauk. Akademiya Nauk Ukrainskoj SSR, pp. 5–170. [Google Scholar]
- Mannani M. 2020. Late Triassic scleractinian corals from Nayband Formation, southwest Ardestan, Central Iran. Boletín la Soc Geológica Mex 72 (2): A090619. [CrossRef] [Google Scholar]
- Mannani M, Yazdi M. 2009. Late Triassic and Early Cretaceous sedimentary sequences of the northern Isfahan Province (Central Iran): stratigraphy and paleoenvironments. Boletín la Soc Geológica Mex 61 (3): 367–374. [CrossRef] [Google Scholar]
- Martin J. 1860. Paléontologie stratigraphique de l’Infralias du département de la Côte d’or suivie d’un aperçu paléontologique sur les mêmes assises dans le Rhône, l’Ardèche et l’Isère. Mémoire la Société Géologique Fr 7: 1–100. [Google Scholar]
- Matthews SC. 1973. Notes on open nomenclature and on synonymy lists. Palaeontology 16 (4): 713–719. [Google Scholar]
- McRoberts C, Blodgett RB. 2002. Late Triassic (Norian) Mollusks from the Taylor Mountains Quadrangle, Southwestern Alaska. In Wilson FH, Galloway J, eds. Studies by the U.S. Geological Survey in Alaska, 2000: U.S. Geological Survey Professional Paper, Vol. 1662, pp. 54–67. [Google Scholar]
- Melnikova GK. 1971. Novye dannye o morfologii, mikrostrukture i sistematike pozdnetriasovykh Thamnasterioidea [New data on morphology, microstructure and systematics of the Late Triassic Thamnasterioidea]. Paleontol Zhurnal 2: 21–35. [Google Scholar]
- Melnikova GK. 1972. Revision of some Late Triassic and Early Jurassic Stylophyllidae (Scleractinia). Palaeontol J 2: 191–200. [Google Scholar]
- Melnikova GK. 1975a. Novye rannejurskie predstaviteli Amphiastraeina (skleraktinii) jugo-vostocnogo pamira. In: Dzalilov V, ed., Akademiya Nauk SSSR. Dushanbe: Akademiya Nauk Tadzikstan SSR, pp. 108–120. [Google Scholar]
- Melnikova GK. 1975b. Pozdnetriasovye scleractinii yugo-vostochnogo Pamira [Late Triassic scleractinians from the southeastern Pamirs]. Dushanbe: Akademiya Nauk Tadzikstan SSR, pp. 1–235. [Google Scholar]
- Melnikova GK. 1984. Novye pozdnetriasovye korally otriada Archaeocoeniida Alloiteau, 1952 yugo-vostochnogo Pamira [New Late Triassic corals of the order Archaeocoeniida Alloiteau, 1952 of the southeast Pamirs]. In: Djalilov MR, ed., Novye Vidy Iskopaemoy Fauny i Flory Tajikistana. Dushanbe: Donisch, pp. 42–55 & 207-208, pl. 18–19. [Google Scholar]
- Melnikova GK. 2001. Coelenterata. In Rozanov AY, Shevyrev AA, eds. Atlas triasovyh bespozvonocnyh Pamira [Atlas of Triassic Invertebrates of the Pamir]. Moscva: Nauka, pp. 30–80. [Google Scholar]
- Melnikova GK. 2006. The Early Jurassic fauna from the Gurumdy and Mynhajir zones of the East Pamirs. Dokl Earth Sci 407: 172–174. [CrossRef] [Google Scholar]
- Melnikova GK, Bychkov YM. 1986. Pozdnetriasovyye skleraktinii khrebta Kenkeren (Koryakskoye nagorye) [Upper Triassic scleractinians from the Kenkeren Ridge (Koryak Range)]. In Zakharov YD, Onopriyenko YI, eds. Korrelyatsiya permo-triasovykh otlozheniy Vostoka SSSR [Correlation of the Permian-Triassic deposits of the eastern USSR]. pp. 63–81. [Google Scholar]
- Melnikova GK, Roniewicz E. 2012. Early Jurassic corals of the Pamir mountains- a new Triassic-Jurassic transitional fauna. Geol Belgica 15: 376–381. [Google Scholar]
- Melnikova GK, Roniewicz E. 2017. Early Jurassic corals with dominating solitary growth forms from the Kasamurg Mountains, Central Asia. Palaeoworld 26 (1): 124–148. [CrossRef] [Google Scholar]
- Melnikova GK, Roniewicz E. 2020. Lower Jurassic corals from the Pamir Mountains, Central Asia. Palaeoworld. [Google Scholar]
- Melnikova GK, Roniewicz E, Löser H. 1993. New microsolenid genus Eocomoseris (Scleractinia, Early Lias-Cenomanian). Ann Soc Geol Pol 63: 3–12, pl. 1–2. [Google Scholar]
- Melnikova and Roniewicz, 2021: MELNIKOVA G.K. & RONIEWICZ E. 2021. — Lower Jurassic corals from the Pamir Mountains, Central Asia. Palaeoworld. 30: 461–494 [Google Scholar]
- Meyer D. 1888. Die Korallen des Doggers von Elsass-Lothringen. Abh Geol Spec Karte Els Lothr Abh 4: 1–44. [Google Scholar]
- Montanaro Gallitelli E. 1975. Hexanthiniaria, a new ordo of Zoantharia (Anthozoa, Coelenterata). Boll della Soc Paleontol Ital 14: 21–25. [Google Scholar]
- Montanaro Gallitelli E, Russo A, Ferrari P. 1979. Upper Triassic Coelenterates of Western North America. Bolletino della Soc Paleontol Ital 18: 133–156. [Google Scholar]
- Morycowa E, Misik M. 2005. Upper Jurassic shallow-water scleractinian corals from the Pieniny Klippen belt (Western Carpathians, Slovakia). Geol Carpathica 56: 415–432. [Google Scholar]
- Morycowa E, Roniewicz E. 1990. Revision of the genus Cladophyllia and description of Apocladophyllia gen.n. (Cladophylliidae fam.n., Scleractinia). Acta Palaeontol Pol 35 (3-4): 165–190, pl. 15–22. [Google Scholar]
- Münster GG. 1841. Beschreibung und Abbildung der in den Kalkmergelschichten von St. Cassian gefundenen Versteinerungen. Beiträge zur Petrefacten-kd 4: 25–39, pl. 1–4. [Google Scholar]
- Negus PE. 1983. Distribution of the British Jurassic corals. Proc Geol Assoc 94 (3): 251–257. [CrossRef] [Google Scholar]
- Negus PE. 1991. Stratigraphical table of scleractinian coral genera and species occurring in the British Jurassic. Proc Geol Assoc 102: 251–259. [CrossRef] [Google Scholar]
- Nicklès R. 1902. Hettangien coralligène de Saint-Félix de l’Héras. Bull des Serv la Cart géologique la Fr des Topogr Souterr 12 (1900-1): 435–436. [Google Scholar]
- Ogilvie MM. 1897. Die Korrallen der Stramberger Schichten. Palaeontographica 7A(Supplement II): 73–282. [Google Scholar]
- Orbigny A d’. 1849. Prodrome de paléontologie stratigraphique universelle des animaux mollusques et rayonnés. Premier volume. Victor Masson, 394 p. [Google Scholar]
- Orbigny A d’. 1850. Prodrome de paléontologie stratigraphique universelle des animaux mollusques et rayonnés. Deuxième volume. Paris: Victor Masson. 428 p. [Google Scholar]
- Pandey DK, Fürsich FT. 1993. Contributions to the Jurassic of Kachchh, Western India. I. The coral fauna. Beringeria 8: 3–69, pl. 1–11. [Google Scholar]
- Pandey DK, Fürsich FT. 2003. Jurassic corals of east-central Iran. Beringeria 32: 3–138. [Google Scholar]
- Pandey DK, Fürsich FT. 2006. Jurassic corals from the Shemshak Formation of the Alborz Mountains, Iran. Zitteliana (41–74) [Google Scholar]
- Pascault O. 2018. Rôle des failles synsédimentaires dans l’implantation et la croissance de mud mounds et de récifs marins en contexte synrift. Cas du Sinémurien terminal du Haut-Atlas marocain. Master Thesis. Université de Bourgogne, 49 p. [Google Scholar]
- Popa E. 1981. La biostratigraphie des formations Mésozoïques de la partie orientale de Pădurea Craiuui (Monts Apuseni). Anu Institutului Geol şi Geofiz. LVIII: 203–282, Pl. I- XXXII. [Google Scholar]
- Poulton TP. 1988. A Lower Jurassic coral reef, Telkwa Range, British Columbia. Can Soc Pet Geol Mem 13: 754–757. [Google Scholar]
- Prinz-Grimm P. 1995. Triassische Korallen der südlichen Zentral-Anden. Geol Palaeontol 29: 233–243. [Google Scholar]
- Prinz P. 1991. Mesozoische Korallen aus nordchile. Palaeontogr Abteilung A Paläozoologie Stratigr 216: 147–209. [Google Scholar]
- R Core Team. 2017. R: A language and environment for statistical computing. [Google Scholar]
- Ramovš A, Turnšek D. 1984. Lower Carnian reef buildups in the Northern Julian Alps (Slovenia, NW Yugoslavia). Razpr IV, Razreda SAZU 25 (4): 163–200. [Google Scholar]
- Reuss AE. 1854. Beiträge zur Charakteristik der Kreideschichten in den Ostalpen, besonders im Gosauthale und am Wolfgangsee. Denkschriften der Kais Akad der Wissenschaften Math Cl 7: 1–156, pl. 1–31. [Google Scholar]
- Reuss AE. 1855. Über zwei Polyparien aus den Hallstätter Schichten. Denkschriften der Kais Akad der Wissenschaften Math Cl 9: 167–169. [Google Scholar]
- Riedel P. 1990. Riffbiotope im Karn und Nor (Obertrias) der Tethys: Entwicklung, Einschnitte und Diversitätsmuster. Doctoral Thesis. Erlangen: Universität Erlangen-Nürnberg, 96 p. [Google Scholar]
- Riedel P. 1991. Korallen in der Trias der Tethys: Stratigraphische Reichweiten, Diversitätsmuster, Entwicklungstren. Mitt Ges Geol Berbaustud Österr 37: 97–118. [Google Scholar]
- Roniewicz E. 1970. Scleractinia from the upper Portlandian of Tisbury, Wiltshire, England. Acta Palaeontol Pol 15 (4): 519–532, pl. 1–4. [Google Scholar]
- Roniewicz E. 1974. Rhaetian corals of the Tatra Moutains. Acta Geol Pol 24 (1): 97–116. [Google Scholar]
- Roniewicz E. 1989. Triassic scleractinian corals of the Zlambach Beds, northern Calcareous Alps, Austria. Denkschriften Österreichische Akad der Wissenschaften, Math Naturwissenschaftliche Klasse 126: 1–152, pl. 1–43. [Google Scholar]
- Roniewicz E. 1996. Upper Triassic solitary corals from the Gosaukamm and other north Alpine regions. Sitzungsberichte der Akad der Wissenschaften Math Klasse 202: 3–41. [Google Scholar]
- Roniewicz E. 2010a. Corallosphere: Spongiomorpha. Available from http://www.corallosphere.org/taxon/1399.html (last consult: 27/11/2020) [Google Scholar]
- Roniewicz E. 2010b. Corallosphere: Pontebbastraea. Available from http://www.corallosphere.org/taxon/1613.html (last consult: 14/04/2021)‘ [Google Scholar]
- Roniewicz E. 2010c. Corallosphere: Coryphylliidae. Available from http://www.corallosphere.org/taxon/1649.html (last consult: 17/11/2023) [Google Scholar]
- Roniewicz E. 2011. Early Norian (Triassic) Corals from the Northern Calcareous Alps, Austria, and the Intra-Norian Faunal Turnover. Acta Palaeontol Pol 56 (2): 401–428. [CrossRef] [Google Scholar]
- Roniewicz E. 2013. Corallosphere: Paravolzeia. Available from http://corallosphere.org/taxon/1828.html (last consult: 05/02/2021) [Google Scholar]
- Roniewicz E, Michalik J. 1998. Rhaetian Scleractinian corals in the western Carpathians. Geol Carpathica 49 (6): 391–399. [Google Scholar]
- Roniewicz E, Michalik J. 2002. Carnian corals from the Male Karpaty Mountains, Western Carpathians, Slovakia. Geol Carpathica 53: 149–157. [Google Scholar]
- Roniewicz E, Morycowa E. 1993. Evolution of the Scleractinia in the light of microstructural data. Cour Forschungsinstitut Senckenb 164: 233–240. [Google Scholar]
- Roniewicz E, Stanley GDJ, Monteiro F da C, Grant-Mackie JA. 2005. Late Triassic (Carnian) corals from Timor-Leste (East Timor): their identity, setting, and biogeography. Alcheringa An Australas. J Palaeontol 29 (2): 287–303. [CrossRef] [Google Scholar]
- Roniewicz E, Stanley GDJ. 2009. Noriphyllia, a new Tethyan Late Triassic coral genus (Scleractinia). Paläontologische Zeitschrift 83: 467–478 [CrossRef] [Google Scholar]
- Rosen BR. 2000. Algal symbiosis, and the collapse and recovery of reef communities, Lazarus corals across the K-T boundary. In Culver SJ, Rawson PF, eds. Biotic Response to Global Change, the Last 145 Million Years. Cambridge: Cambridge University Press, pp. 164–180. [Google Scholar]
- Rosenblatt MR. 2010. Upper triassic corals and carbonate reef facies from the martin bridge and hurwal formations, wallowa terrane (Oregon). Master Thesis. USA: The University of Montana, 152 p. [Google Scholar]
- Sadati S. 1981. Die Hohe Wand: Ein obertriadisches Lagunen-Riff am Ostende der Nördlichen Kalkalpen (Nordösterreich). Facies 5: 191–264. [CrossRef] [Google Scholar]
- Sarih S. 2008. Géodynamique et transferts sédimentaires gravitaires des bassins liasiques du Haut-Atlas central (Maroc). Université de Bourgogne, 301 p. [Google Scholar]
- Sarih S, Quiquerez A, Allemand P, Garcia J-P., El Hariri K. 2018. Along strike behavior of the Tizi n’ Firest fault during the Lower Jurassic rifting (Central High Atlas Carbonate basin, Morocco). Int J Earth Sci 107 (6): 2209–2231. [CrossRef] [Google Scholar]
- Schäfer P. 1979. Fazielle Entwicklung und Palökologische Zonierung Zweier Obertriadischer Riffstrukturen in den Nördlichen Kalkalpen (“Oberrhät”-Riff-Kalke, Salzburg). Facies 1 (1): 3–245. [CrossRef] [Google Scholar]
- Schlippe AO. 1888. Die Fauna des Bathon im oberrheinischen Tiefland. Abh Geol Spec Karte Els Lothr 4: 1–226. [Google Scholar]
- Schlüter C. 1889. Anthozoen des rheinischen Mittel-Devon. Abhandlungen zur Geol Spec von Preuss und den Thüringischen Staaten 8 (4): 259–465, pl. 1–16. [Google Scholar]
- Senowbari-Daryan B. 1980. Fazielle und paläontologische Untersüchungen in Oberrhätischen riffen (Feichtenstein und Gruberriff). Facies 3: 1–237. [CrossRef] [Google Scholar]
- Senowbari-Daryan B. 1996. Upper Triassic Reefs and Reef communities of Iran. Göttinger Arb zur Geol und Paläontologie Sb 2: 299–304. [Google Scholar]
- Senowbari-Daryan B, Stanley GDJ. 1994. Lower jurassic marine carbonate deposits in central Peru: stratigraphy and paleontology. Palaeontogr Abteilung A Paläozoologie, Stratigr 233: 43–56. [Google Scholar]
- Shepherd HME, Stanley GDJ, Amirhassankhani F. 2012. Norian to Rhaetian scleractinian corals in the Ferdows Patch Reef (Nayband Formation, east central Iran). J Paleontol 86 (5): 801–812. [CrossRef] [Google Scholar]
- Simms MJ, Little CTS, Rosen BR. 2002. Corals not serpulids: mineralized colonial fossils in the Lower Jurassic marginal facies of South Wales. Proc Geol Assoc 113 (1): 31–36. [CrossRef] [Google Scholar]
- Smith JP. 1927. Upper Triassic marine invertebrate faunas of North America. United States Geol Surv Prof Pap 141: 1–262, pl. 1–121. [Google Scholar]
- Squires DF. 1956. A new Triassic Coral Fauna from Idaho. Geol Soc Am Spec Pap 1797: 1–27. [Google Scholar]
- Stanley GDJ. 1979. Paleoecology, structure, and distribution of Triassic coral buildups in Western North America. Univ Kansas, Paleontol Contrib 65: 1–58. [Google Scholar]
- Stanley GDJ. 1986. Late Triassic coelenterate faunas of western Idaho and northeastern Oregon: Implications for biostratigraphy and paleogeography. US Geol Surv Prof Pap 1435: 23–39. [Google Scholar]
- Stanley GDJ. 1989. An Upper Triassic reefal limestone, southern Vancouver Island, B.C. In Geldsetzer HHJ, James NP, eds. Canadian Society of Petroleum Geologists, Memoirs, Vol. V 13, pp. 766–776. [Google Scholar]
- Stanley GDJ. 1992. Tropical reef ecosystems and their evolution. In: Nierenberg WA, ed. Encyclopedia of earth system science. Academic Press, San Diego, Vol. 4. [Google Scholar]
- Stanley GDJ. 1994. Upper Triassic corals from Peru. Palaeontogr Abteilung A Paläozoologie, Stratigr 233: 75–98. [Google Scholar]
- Stanley GDJ. 1997. Evolution of reefs of the Mesozoic. Proc 8th Int Coral reef Sym 2: 1657–1662. [Google Scholar]
- Stanley GDJ. 2001. Introduction to reef ecosystems and their evolution BT − the history and sedimentology of ancient reef systems. In: Stanley GD, ed. Boston, MA: Springer US, pp. 1–39. [Google Scholar]
- Stanley GDJ, Beauvais L. 1994. Corals from an Early Jurassic coral reef in British Columbia: refuge on an oceanic island reef. Lethaia 27: 35–47. [CrossRef] [Google Scholar]
- Stanley GDJ, McRoberts CA. 1993. A coral reef in the Telkwa Range, British Columbia: the earliest Jurassic example. Can J Earth Sci 30 (4): 819–831. [CrossRef] [Google Scholar]
- Stanley GDJ, Onoue T. 2015. Upper Triassic reef corals from the Sambosan Accretionary Complex, Kyushu, Japan. Facies 61 (2): 1–27. [CrossRef] [Google Scholar]
- Stanley GDJ, Senowbari-Daryan B. 1986. Upper Triassic, Dachstein-Type, Reef Limestone from the Wallowa Mountains, Oregon: First Reported Occurrence in the United States. Palaios 1 (2): 172–177. [CrossRef] [Google Scholar]
- Stanley GDJ, Whalen MT. 1989. Triassic corals and spongiomorphs from Hells Canyon, Wallowa Terrane, Oregon. J Paleontol 63 (6): 800–819. [CrossRef] [Google Scholar]
- Stanley GDJ, Yarnell JM. 2003. New paleontological investigations of the Triassic carbonate rocks in the Upper Chulitna District (Chulitna terrane), southcentral Alaska. In Clautice KH, Davies PK, eds. Short Notes on Alaska Geology 2003. Professional Report of the Division of Geological and Geophysical Surveys Alaska Vol. V 120, pp. 109–116. [CrossRef] [Google Scholar]
- Stolarski J, Roniewicz E. 2001. Towards a new synthesis of evolutionary relationships and classification of Scleractinia. J Paleontol 75 (6): 1090–1108. [CrossRef] [Google Scholar]
- Stolarski J, Russo A. 2002. Microstructural diversity of the stylophyllid (Scleractinia) skeleton. Acta Palaeontol Pol 47 (4): 651–666. [Google Scholar]
- Stone T, Martindale RC, Fonville T, et al. 2022. Assessment of a reef community from Lower Jurassic (Pliensbachian) strata in the Central High Atlas Mountains of Morocco. Palaios 37 (11): 633–649. [CrossRef] [Google Scholar]
- Straw SH. 1925. On some english species of the genus Stylophyllopsis. Palaeontogr Soc Monogr 62: 350–363. [Google Scholar]
- Terquem O, Piette E. 1865. Le Lias inférieur de l’Est de la France, comprenant le Meurthe, la Moselle, le Grand-Duché de Luxembourg, la Belgique et la Meuse. 3: 1–176. [Google Scholar]
- Terquem O, Piette E. 1868. Le Lias inférieur de l’Est de la France. Ann des Sci Nat − 3ième Série 8: 1–175. [Google Scholar]
- Tilmann N. 1917. Die Fauna des unteren und mittleren Lias in Nord und Mittel-Peru. Neues Jahrb für Mineral Geol und Paläontologie 41: 628–712. [Google Scholar]
- Tomes RF. 1878. On the stratigraphical position of the corals of the Lias of the Midland and western counties of England and of South Wales. Q J Geol Soc 34 (1-4): 179–195, pl. 9. [CrossRef] [Google Scholar]
- Tomes RF. 1882. On the madreporaria of the inferior Oolite of the neighborhood of Cheltenham and Gloucester. Q J Geol Soc London 38: 409–450. [CrossRef] [Google Scholar]
- Tomes RF. 1884a. A critical and descriptive list of the oolitic madreporaria of the boulonnais. Q J Geol Soc 40 (1-4): 698–723. [CrossRef] [Google Scholar]
- Tomes RF. 1884b. A comparative and critical revision of the madreporaria of the white lias of the middle and western counties of England, and of those of the conglomerate at the base of the South-Wales Lias. Q J Geol Soc 40 (1-4): 353–375. [CrossRef] [Google Scholar]
- Tomes RF. 1888. II.—On Heterastræa, a new Genus of Madreporia from the Lower Lias. Geol Mag 5 (5): 207–218. [CrossRef] [Google Scholar]
- Tomes RF. 1890. Notes on an annotated (ou amended?) list of madreporaria of Crickley Hill. Cotteswold Nat Field Club Proc 9: 300–307. [Google Scholar]
- Tomes RF. 1893. Observations on the affinities of the genus Astrocoenia. Q J Geol Soc 49 (1-4): 569–573, pl. 20. [CrossRef] [Google Scholar]
- Tornquist A. 1899. III. Neue Beiträge zur Geologie und Paläontologie der Umgebung von Recoaro und Schio (im Vicentin). Zeitschrift der Dtsch Geol Gesellschaft Bd. 51 (Heft 3): 341–377. [Google Scholar]
- Turnšek D. 1997. Mesozoic corals of Slovenia. Ljubljana: Znanstvenoraziskovalni Center SAZU, 512 p. [Google Scholar]
- Turnšek D, Buser S. 1989. The Carnian reef complex on the Pokljuka (NW Yugoslavia). Razpr IV, Razreda SAZU 30: 75–127. [Google Scholar]
- Turnšek D, Buser S. 1991. Norian-Rhaetian coral reef buildups in Bohinj and Rdeči Rob in southern Julian Alps (Slovenia). Razpr IV, Razreda SAZU 32: 215–257. [Google Scholar]
- Turnšek D, Buser S. 1999. Stylophyllopsis veneta (Airaghi), a Liassic coral from the northern Dinaric Carbonate Platform (Slovenia). Profil 16: 173–180. [Google Scholar]
- Turnšek D, Buser S, Debeljak I. 2003. Liassic coral patch reef above the“ Lithiotid limestone” on Trnovski gozd plateau, west Slovenia: Liasni koralni kopasti greben na” litiotidnem apnencu” v Trnovskem gozdu, zahodna Slovenija. Razpr IV Razreda Sazu 44 (1): 285–331. [Google Scholar]
- Turnšek D, Kosir A. 2000. Early Jurassic corals from Krim Montain, Slovenia. Razpr IV, Razreda SAZU 41 (1): 81–113. [Google Scholar]
- Turnšek D, Ramovš A. 1987. Upper Triassic (Norian-Rhaetian) reef buildups in the northern Julian Alps (NW Yugoslavia). Razpr IV, Razreda SAZU 28 (2): 27–67. [Google Scholar]
- Turnšek D, Senowbari-Daryan B. 1994. Upper Triassic (Carnian-Lowermost Norian) corals from the Pantokrator Limestone of Hydra (Greece). Abhandlungen der Geol Bundesanstalt 50: 477–507. [Google Scholar]
- Turnšek D, Seyfried H, Geyer OF. 1975. Geologische und paleontologische Untersuchungen an einem Korallen-Vorkommen im subbetischen Unterjura von Murcia (Süd-Spanien). Razpr IV, Razreda SAZU 18 (5): 117–151. [Google Scholar]
- Vasseur R, Lathuilière B, Lazăr I, Martindale RC, Bodin S, Durlet C. 2021. Major coral extinctions during the early Toarcian global warming event. Glob Planet Change 207: 103647. [CrossRef] [Google Scholar]
- Vasseur R. 2018. Extinctions et recouvrements de coraux au cours de la crise Pliensbachien − Toarcien. Université de Lorraine, 542 p. [Google Scholar]
- Vasseur R, Boivin S, Lathuilière B, et al. 2019. Lower Jurassic corals from Morocco with skeletal structures convergent with those of Paleozoic rugosan corals. Palaeontol Electron 22 (2). [CrossRef] [Google Scholar]
- Vasseur R, Lathuilière B. 2021. Pliensbachian corals from the Western Tethys. Geodiversitas 43 (22): 1187–1291. [CrossRef] [Google Scholar]
- Vaughan TW, Wells JW. 1943. Revision of the suborders, families, and genera of the Scleractinia. Geol Soc Am Spec Pap 44: 1–345, pl. 1–51. [Google Scholar]
- Verrill AE. 1864. List of the polyps and corals sent by the Museum of Comparative Zoology to other institutions in exchange, with annotations. Bull Mus Comp Zool 1: 29–60. [Google Scholar]
- Vinassa de Regny P. 1911. Triadische Algen, Spongien, Anthozoen und Bryozoen aus Timor. Paläontologie von Timor 4: 75–118. [Google Scholar]
- Volz W. 1896. Die Korallenfauna der Trias. Monographisch bearbeitet von Fritz Frech und Wilhelm Volz. II. Die Korallen der Schichten von St. Cassian in Süd-Tirol. Palaeontographica 43: 1–123, pl. 1–11. [Google Scholar]
- Wells JW. 1956. Scleractinia. In: Moore RC, ed., Treatise on invertebrate paleontology. Lawrence, KS: Geological Society of America & University of Kansas Press, Vol. F pp. 328–444. [Google Scholar]
- Wells JW. 1936. The nomenclature and type species of some genera of Recent and fossil corals. Am J Sci 31 (182): 97–134. [CrossRef] [Google Scholar]
- Wells JW. 1943. Paleontology of the Harrar Province, Ethiopia. Part 3. Jurassic Anthozoa and Hydrozoa. Bull Am Museum Nat Hist 82 (2): 31–54, pl. 5–9. [Google Scholar]
- Wells JW. 1953. Mesozoic invertebrate faunas of Peru. Part 3, Lower Jurassic corals from the Arequipa region. Am Museum Novit 1631: 1–14. [Google Scholar]
- Wickham H. 2009. ggplot2: elegant graphi graphi cs for data analysis. Springer-Verlag New York. [CrossRef] [Google Scholar]
- Winkler GG. 1861. Der Oberkeuper, nach Studien in den bayrischen Alpen. Zeitschrift der Dtsch Geol Gesellschaft 13: 459–521. [Google Scholar]
- Wu W. 1975. The coral fossils from the Qomolangma Feng region., Report of Scientific Investigations in the Qomolongma Feng Region (Paleontology Fasc. I). Beijing: Nanking Institute of Geology and Paleontology, Academia Sinica, pp. 83–113. [Google Scholar]
- Wu W. 1977. Triassic Scleractinia corals from NW Yunnan. Mesozoic Fossils of Yunnan. Vol. 2, pp. 29–37. [Google Scholar]
- Xia J, Liao W. 1986. Some Scleractinian Corals of Procyclolitidae from Lhasa. Acta Palaeontol Sin 25: 37–47. [Google Scholar]
Cite this article as: Boivin S, Lathuilière B, Martini R. 2025. Coral biodiversity from Morocco after the End-Triassic mass extinction, BSGF - Earth Sciences Bulletin 196: 6. https://doi.org/10.1051/bsgf/2024027
All Tables
Summary table of outcrops sampled in the Amellagou Region, High Atlas Mountains, Morocco.
List of genera and species with their distribution by localities and colonial structures. The numbers correspond to the numbers of specimens and brackets to doubtful assignations.
Comparisons between dimensions of Lepidophyllia (Heterastraea) nominal species. Two first measurements are in millimetres and rounded to the nearest half-millimetre. D diameter, CC distance from calice to calice, NS number of septa, Sd septal density. The dimensions are those from: 1 Beauvais (1976), 2 Turnšek & Kosir (2000), 3 Turnšek, Buser, & Debeljak (2003), 4 Gretz (2014), 5 Tomes (1888), 6 Terquem & Piette (1865), 7 Fromentel (1860).
Dimensions of type material of Stylophyllopsis nominal species. Diameters are rounded to the nearest half-millimetre. F. Dimensions of figured material in absence of type specimen (S. guettardi: figuration from Lejeune, 1935). H. Dimensions of the holotype. L Dimensions of the lectotype. TS. Dimensions of the type series. S Dimensions of the syntype(s). * Dimensions estimated from figures or photographies. Remark: Stylophyllopsis pontebbanae Volz, 1896 is not included in the table because it has been reassigned to Pontebbastraea Roniewicz & Michalik, 200; 2, a junior synonym of Remismilia Beauvais, 1972 according to Roniewicz, 2010b. Stylophyllopsis laxa (Fromentel & Ferry, 1867) is considered a junior synonym of S. fritillus according to Vasseur & Lathuilière (2021). S. rugosa (Duncan, 1868) is regarded as a junior synonym of S. walliae (Duncan, 1867) according to Beauvais (1986).
All Figures
 |
Fig. 1 Map of the main structural domains of Morocco with location of Amellagou Region in High Atlas Mountains. Modified after Krencker et al. (2014). |
| In the text | |
 |
Fig. 2 Illustration of the main values measured for the morphometric study of corals. The black area corresponds to the skeleton. D calicular great diameter, d calicular small diameter, tw thickness of the wall, cc distance from corallite to corallite. |
| In the text | |
 |
Fig. 3 Hispaniastraea murciana Turnšek & Geyer, 1975. A Natural transverse section of the specimen SB–243. B Transverse thin section of a branch of the specimen SB–246. C–D Longitudinal thin sections of branches of the specimen SB–246. |
| In the text | |
 |
Fig. 4 Hispaniastraea murciana Turnšek & Geyer, 1975. A Transverse thin section of a branch of the specimen SB–54. B Transverse thin section a branch of the specimen SB–85. C Longitudinal thin section of the specimen SB–169. D Longitudinal thin section of a branch of the specimen SB–53. |
| In the text | |
 |
Fig. 5 Chondrocoenia clavellata (Terquem & Piette, 1865). A Specimen SB–194. B Enlargement of A. C Longitudinal thin section of branch of the specimen SB–49. D Enlargement of C. |
| In the text | |
 |
Fig. 6 Chondrocoenia martini (Fromentel, 1860). A Natural view of a calice of the specimen SB–343. B Transverse thin section of the specimen SB–343. C Enlargement of B. D Transverse thin section of the specimen SB–157. |
| In the text | |
 |
Fig. 7 Lochmaeosmilia? sp. Transverse thin section of the specimen SB–155. |
| In the text | |
 |
Fig. 8 Parastraeomorpha minuscula Roniewicz, 1989. A Transverse thin section of the specimen SB–344. B–C–D Enlargement of A. |
| In the text | |
 |
Fig. 9 Eocomoseris minima (Beauvais, 1986). A Longitudinal thin section of a branch of the specimen SB–222. B Enlargement of A. C Transverse thin section of branches of the specimen SB–245. D–E Enlargement of C. F Longitudinal thin sections of branches of the specimen SB–245. |
| In the text | |
 |
Fig. 10 Eocomoseris minima (Beauvais, 1986). A Longitudinal thin section of the specimen SB–167/2. B Longitudinal thin section of the specimen SB–316/2. |
| In the text | |
 |
Fig. 11 Araiophyllum triasicum (Cuif, 1975b). Transverse thin section of the specimen SB–242/2. |
| In the text | |
 |
Fig. 12 Thecactinastraea fasciculata Beauvais, 1986. A–C Transverse thin sections of the specimen SB–153. D Oblique thin section of the specimen SB–80. E Enlargement of D. F Oblique thin section of the specimen SB–80. |
| In the text | |
 |
Fig. 13 Thecactinastraea termierorum (Beauvais, 1986). A–B Transverse thin section of the specimen SB–379. C Longitudinal thin section of the specimen SB–200. D–E Longitudinal thin section of the specimen SB–55/3. F Transverse thin section of the specimen SB–55/3. |
| In the text | |
 |
Fig. 14 Thecactinastraea termierorum (Beauvais, 1986). A–D Transverse thin sections of branches of the specimen SB–62. |
| In the text | |
 |
Fig. 15 Coryphyllia capillaria Vasseur & Lathuilière, 2021. A Transverse thin section of the specimen SB–244. B–C Longitudinal thin section of the specimen SB–230/1, section B distal and section C proximal. D Longitudinal thin section of the specimen SB–214. E Transverse thin section of the specimen SB–214. F Enlargement of E. Note the mid-septal line. |
| In the text | |
 |
Fig. 16 Coryphyllia regularis Cuif, 1975a. A Transverse thin section of the specimen SB–69. B Transverse thin section of the specimen SB–146/1. C Transverse thin section of the specimen SB–316/1. D Transverse thin section of the specimen SB–242/1. |
| In the text | |
 |
Fig. 17 Coryphyllia subregularis ? Beauvais, 1986. Oblique thin section of the specimen SB–169/2. |
| In the text | |
 |
Fig. 18 Paracuifia castellum sp. nov. A & E transverse section of branches of the holotype SB–78/1. B–D longitudinal section of branches of the holotype SB–78/1. F Enlargement of E. |
| In the text | |
 |
Fig. 19 Proleptophyllia granulum (Fromentel & Ferry, 1866). A Transverse thin section of the specimen SB–342. B Enlargement of A showing the septal ornamentation. C Distal transverse section of the specimen SB–167/1. Proleptophyllia granulum? (Fromentel and Ferry, 1866). D Transverse section of the badly preserved specimen SB–326/1. |
| In the text | |
 |
Fig. 20 Periseris elegantula (Orbigny, 1850). A Transverse thin section of the specimen SB–345. B Enlargement of A. C Longitudinal thin section of the specimen SB–345. D–E Enlargements of C. F Transverse thin section of the specimen SB–237. G Enlargement of F. |
| In the text | |
 |
Fig. 21 Paravolzeia? alpina? Roniewicz et al., 2005. Transverse thin section of the specimen SB–219/2. |
| In the text | |
 |
Fig. 22 Distichophyllia norica (Frech, 1890). A Transverse thin section of three contiguous corallites (specimens SB–202). B Enlargement of A. C Transverse thin section of the specimen SB–159. D Transverse thin section of the specimen SB–165. E Transverse thin section of the specimen SB–203. F Enlargement of E. |
| In the text | |
 |
Fig. 23 Retiophyllia? cf. gracilis Roniewicz, 1989. A–B Transverse thin sections of two branches of the specimen SB–204/2. C. Enlargement of B. |
| In the text | |
 |
Fig. 24 Epismiliopsis sp. A Transverse thin section of the specimen SB–55/2. B Enlargement of A. C Transverse thin section of the specimen SB–331. D Enlargement of C. |
| In the text | |
 |
Fig. 25 Lepidophyllia (Heterastraea) microcalix sp. nov. A Transverse section of the colony SB–48. B Transverse section of the colony SB–204/1. C–D Transverse sections of the colony SB–210/1. G Transverse section of the colony SB–329. F Transverse section of the colony SB–211. G transverse section of the colony SB–318/1. |
| In the text | |
 |
Fig. 26 Lepidophyllia (Heterastraea) microcalix sp. nov. A–B longitudinal sections of the colony SB–329. Note the two opposite septa cut longitudinally in the centre of the corallite and the ornamentation of their inner margins (A). C longitudinal section of the colony SB–228. D longitudinal section of the holotype SB–220. E–F transverse sections of the colony SB–216, note the septal intracalicular budding. |
| In the text | |
 |
Fig. 27 Plots of morphometrical measurements of Lepidophyllia (Heterastraea) microcalix sp. nov. A Histograms of morphometrical measurements (calicular great diameter, calicular small diameter, thickness of the wall, distance from corallite to corallite, number of septa and septal density). B Morphospace plot of corallites from studied colonies computed with PCA (co-variance matrix) on the two calicular diameters (great and small), thickness of the wall and distance from corallite to corallite. Each dot color represents one colony. The two first principal components explain 92.9% of the total variance. |
| In the text | |
 |
Fig. 28 Plot of dimensions for Lepidophyllia (Heterastraea) nominal species presented in Table 6. |
| In the text | |
 |
Fig. 29 Morphospace plot of Lepidophyllia (Heterastraea) nominal species computed with PCA (co-variance matrix) on the dimensions from litterature presented in Table 6: calicular diameters (Dmin minimum value and Dmax maximum value), distance from corallite to corallite (CCmin minimum value and CCmax maximum value), number of septa (NSmin minimum value and NSmax maximum value) and septal density (SDmin minimum value and SDmax maximum value). The two first principal components explain 77.8% of total variance. |
| In the text | |
 |
Fig. 30 Phacelostylophyllum mg. martini (Fromentel, 1860). A Transverse thin section of a branch of the specimen SB–205. B Longitudinal slightly oblique thin section of a branch of the specimen SB–231. |
| In the text | |
 |
Fig. 31 Phacelostylophyllum mg. suttonensis (Duncan, 1867). A–B Transverse thin sections of branches of the specimen SB–170. |
| In the text | |
 |
Fig. 32 Pinacophyllum sp. A Transverse thin section of the specimen SB–78/2. B Oblique thin section of the specimen SB–78/2. C Transverse thin section of the specimen SB–60. D Oblique thin section of the specimen SB–60. |
| In the text | |
 |
Fig. 33 –Stylophyllopsis cf. walliae (Duncan, 1867). Transverse section of specimen SB–207. |
| In the text | |
 |
Fig. 34 Plots of dimensions of the type material of Stylophyllopsis nominal species presented in Table 7. |
| In the text | |
 |
Fig. 35 Vallimeandropsis davidsoni (Edwards & Haime, 1851a) A Transverse thin section of the colony SB–335. B–C Enlargements of A. D Longitudinal thin section of the colony SB–143. E Transverse thin section of the colony SB–143. |
| In the text | |
 |
Fig. 36 Gen. indet. sp. A transverse section of specimen SB–210/2. B–C Enlargement of A. |
| In the text | |
 |
Fig. 37 Neorylstonia pseudocolumellata (Beauvais, 1986) A Natural transverse section of the specimen SB–166. B Natural transverse section of the specimen SB–236. C–D Transverse thin section of the specimen SB–236. Section C is more distal (12 mm) than section D. |
| In the text | |
 |
Fig. 38 Rarefaction curves of the taxa from the different studied localities (i.e., the Dromedary biostrome, the Serdrar reef, the Castle olistolith and the Owl olistolith), in comparison to the global curve that takes into account the whole set of assemblages described in the present study. |
| In the text | |
 |
Fig. 39 A Distribution of collected specimens from the different studied localities (i.e., the Dromedary biostrome, the Serdrar reef, the Castle olistolith and the Owl olistolith), according to their colonial arrangement. B Distribution of collected species from the different studied localities according to their colonial arrangement. |
| In the text | |
 |
Fig. 40 A Distribution of collected specimens according to their colonial arrangement and split by their different studied localities (i.e., the Dromedary biostrome, the Serdrar reef, the Castle olistolith and the Owl olistolith), B Distribution of collected species from the different studied localities, according to their colonial arrangement t and spitted by their different studied localities. |
| In the text | |
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.




